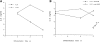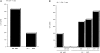Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells - PubMed (original) (raw)
Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells
M Rincón et al. J Exp Med. 1997.
Abstract
Interleukin (IL)-4 is the most potent factor that causes naive CD4+ T cells to differentiate to the T helper cell (Th) 2 phenotype, while IL-12 and interferon gamma trigger the differentiation of Th1 cells. However, the source of the initial polarizing IL-4 remains unclear. Here, we show that IL-6, probably secreted by antigen-presenting cells, is able to polarize naive CD4+ T cells to effector Th2 cells by inducing the initial production of IL-4 in CD4+ T cells. These results show that the nature of the cytokine (IL-12 or IL-6), which is produced by antigen-presenting cells in response to a particular pathogen, is a key factor in determining the nature of the immune response.
Figures
Figure 1
IL-6 directs differentiation of CD4+ T cells into Th2 cells. (A) Total CD4+ T cells (106/ml) were purified from normal B10.BR mice and stimulated (in the presence of 5 × 105 cell/ml syngeneic APCs) with Con A (2.5 μg/ml) alone, Con A plus IL-4 (103 U/ml) or Con A plus IL-12 (3.5 ng/ml), in the absence (−) or presence of IL-6 (100 ng/ml) or IL-2 (50 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cells/ml) with Con A alone (2.5 μg/ml) in the absence of APCs or additional cytokines. Supernatants were harvested 24 h later and analyzed for IL-4 and IFN-γ production by ELISA. (B) Total CD4+ population was isolated from Cyt c TCR transgenic mice, and the naive CD4+ CD45RBhighCD44low subpopulation was purified by cell sorting. Naive CD4+ T cells were then stimulated with Con A alone, Con A plus IL-4, or Con A plus IL-12, and APCs, in the presence or absence of IL-6. After 4 d, cells were exhaustively washed and restimulated (106 cell/ ml) with Con A alone for 24 h before harvesting the supernatants for cytokine measurement. (C) Naive CD4+CD45RBhighCD44low CD4+ T cells isolated from Cyt c TCR transgenic mice were stimulated with Cyt c peptide and APCs, in the presence of medium, IL-6 (100 ng/ml), or IL-4, (103 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cell/ml) with Cyt c peptide and APCs for 24 h. (D) Total CD4+ T cells from normal B10.BR mice were stimulated with immobilized anti– CD3 mAb (5 μg/ml) and soluble anti–CD28 (1 μg/ml) in the presence of medium (−), IL-6 (100 ng/ml), or IL-4 (103 U/ml) and, after 4 d, they were restimulated with immobilized anti–CD3 mAb. (E) Total CD4+ T cells from normal B10.BR mice were stimulated with Con A and APCs in the presence of medium (−), IL-6 (100 ng/ml) (IL-6), or IL-6 (100 ng/ml) plus anti–IL-4 mAb (10 μg/ml) (IL-6 + anti–IL-4), for 4 d. Cells were then restimulated (106 cells/ml) with Con A alone as described in A.
Figure 1
IL-6 directs differentiation of CD4+ T cells into Th2 cells. (A) Total CD4+ T cells (106/ml) were purified from normal B10.BR mice and stimulated (in the presence of 5 × 105 cell/ml syngeneic APCs) with Con A (2.5 μg/ml) alone, Con A plus IL-4 (103 U/ml) or Con A plus IL-12 (3.5 ng/ml), in the absence (−) or presence of IL-6 (100 ng/ml) or IL-2 (50 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cells/ml) with Con A alone (2.5 μg/ml) in the absence of APCs or additional cytokines. Supernatants were harvested 24 h later and analyzed for IL-4 and IFN-γ production by ELISA. (B) Total CD4+ population was isolated from Cyt c TCR transgenic mice, and the naive CD4+ CD45RBhighCD44low subpopulation was purified by cell sorting. Naive CD4+ T cells were then stimulated with Con A alone, Con A plus IL-4, or Con A plus IL-12, and APCs, in the presence or absence of IL-6. After 4 d, cells were exhaustively washed and restimulated (106 cell/ ml) with Con A alone for 24 h before harvesting the supernatants for cytokine measurement. (C) Naive CD4+CD45RBhighCD44low CD4+ T cells isolated from Cyt c TCR transgenic mice were stimulated with Cyt c peptide and APCs, in the presence of medium, IL-6 (100 ng/ml), or IL-4, (103 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cell/ml) with Cyt c peptide and APCs for 24 h. (D) Total CD4+ T cells from normal B10.BR mice were stimulated with immobilized anti– CD3 mAb (5 μg/ml) and soluble anti–CD28 (1 μg/ml) in the presence of medium (−), IL-6 (100 ng/ml), or IL-4 (103 U/ml) and, after 4 d, they were restimulated with immobilized anti–CD3 mAb. (E) Total CD4+ T cells from normal B10.BR mice were stimulated with Con A and APCs in the presence of medium (−), IL-6 (100 ng/ml) (IL-6), or IL-6 (100 ng/ml) plus anti–IL-4 mAb (10 μg/ml) (IL-6 + anti–IL-4), for 4 d. Cells were then restimulated (106 cells/ml) with Con A alone as described in A.
Figure 1
IL-6 directs differentiation of CD4+ T cells into Th2 cells. (A) Total CD4+ T cells (106/ml) were purified from normal B10.BR mice and stimulated (in the presence of 5 × 105 cell/ml syngeneic APCs) with Con A (2.5 μg/ml) alone, Con A plus IL-4 (103 U/ml) or Con A plus IL-12 (3.5 ng/ml), in the absence (−) or presence of IL-6 (100 ng/ml) or IL-2 (50 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cells/ml) with Con A alone (2.5 μg/ml) in the absence of APCs or additional cytokines. Supernatants were harvested 24 h later and analyzed for IL-4 and IFN-γ production by ELISA. (B) Total CD4+ population was isolated from Cyt c TCR transgenic mice, and the naive CD4+ CD45RBhighCD44low subpopulation was purified by cell sorting. Naive CD4+ T cells were then stimulated with Con A alone, Con A plus IL-4, or Con A plus IL-12, and APCs, in the presence or absence of IL-6. After 4 d, cells were exhaustively washed and restimulated (106 cell/ ml) with Con A alone for 24 h before harvesting the supernatants for cytokine measurement. (C) Naive CD4+CD45RBhighCD44low CD4+ T cells isolated from Cyt c TCR transgenic mice were stimulated with Cyt c peptide and APCs, in the presence of medium, IL-6 (100 ng/ml), or IL-4, (103 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cell/ml) with Cyt c peptide and APCs for 24 h. (D) Total CD4+ T cells from normal B10.BR mice were stimulated with immobilized anti– CD3 mAb (5 μg/ml) and soluble anti–CD28 (1 μg/ml) in the presence of medium (−), IL-6 (100 ng/ml), or IL-4 (103 U/ml) and, after 4 d, they were restimulated with immobilized anti–CD3 mAb. (E) Total CD4+ T cells from normal B10.BR mice were stimulated with Con A and APCs in the presence of medium (−), IL-6 (100 ng/ml) (IL-6), or IL-6 (100 ng/ml) plus anti–IL-4 mAb (10 μg/ml) (IL-6 + anti–IL-4), for 4 d. Cells were then restimulated (106 cells/ml) with Con A alone as described in A.
Figure 1
IL-6 directs differentiation of CD4+ T cells into Th2 cells. (A) Total CD4+ T cells (106/ml) were purified from normal B10.BR mice and stimulated (in the presence of 5 × 105 cell/ml syngeneic APCs) with Con A (2.5 μg/ml) alone, Con A plus IL-4 (103 U/ml) or Con A plus IL-12 (3.5 ng/ml), in the absence (−) or presence of IL-6 (100 ng/ml) or IL-2 (50 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cells/ml) with Con A alone (2.5 μg/ml) in the absence of APCs or additional cytokines. Supernatants were harvested 24 h later and analyzed for IL-4 and IFN-γ production by ELISA. (B) Total CD4+ population was isolated from Cyt c TCR transgenic mice, and the naive CD4+ CD45RBhighCD44low subpopulation was purified by cell sorting. Naive CD4+ T cells were then stimulated with Con A alone, Con A plus IL-4, or Con A plus IL-12, and APCs, in the presence or absence of IL-6. After 4 d, cells were exhaustively washed and restimulated (106 cell/ ml) with Con A alone for 24 h before harvesting the supernatants for cytokine measurement. (C) Naive CD4+CD45RBhighCD44low CD4+ T cells isolated from Cyt c TCR transgenic mice were stimulated with Cyt c peptide and APCs, in the presence of medium, IL-6 (100 ng/ml), or IL-4, (103 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cell/ml) with Cyt c peptide and APCs for 24 h. (D) Total CD4+ T cells from normal B10.BR mice were stimulated with immobilized anti– CD3 mAb (5 μg/ml) and soluble anti–CD28 (1 μg/ml) in the presence of medium (−), IL-6 (100 ng/ml), or IL-4 (103 U/ml) and, after 4 d, they were restimulated with immobilized anti–CD3 mAb. (E) Total CD4+ T cells from normal B10.BR mice were stimulated with Con A and APCs in the presence of medium (−), IL-6 (100 ng/ml) (IL-6), or IL-6 (100 ng/ml) plus anti–IL-4 mAb (10 μg/ml) (IL-6 + anti–IL-4), for 4 d. Cells were then restimulated (106 cells/ml) with Con A alone as described in A.
Figure 1
IL-6 directs differentiation of CD4+ T cells into Th2 cells. (A) Total CD4+ T cells (106/ml) were purified from normal B10.BR mice and stimulated (in the presence of 5 × 105 cell/ml syngeneic APCs) with Con A (2.5 μg/ml) alone, Con A plus IL-4 (103 U/ml) or Con A plus IL-12 (3.5 ng/ml), in the absence (−) or presence of IL-6 (100 ng/ml) or IL-2 (50 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cells/ml) with Con A alone (2.5 μg/ml) in the absence of APCs or additional cytokines. Supernatants were harvested 24 h later and analyzed for IL-4 and IFN-γ production by ELISA. (B) Total CD4+ population was isolated from Cyt c TCR transgenic mice, and the naive CD4+ CD45RBhighCD44low subpopulation was purified by cell sorting. Naive CD4+ T cells were then stimulated with Con A alone, Con A plus IL-4, or Con A plus IL-12, and APCs, in the presence or absence of IL-6. After 4 d, cells were exhaustively washed and restimulated (106 cell/ ml) with Con A alone for 24 h before harvesting the supernatants for cytokine measurement. (C) Naive CD4+CD45RBhighCD44low CD4+ T cells isolated from Cyt c TCR transgenic mice were stimulated with Cyt c peptide and APCs, in the presence of medium, IL-6 (100 ng/ml), or IL-4, (103 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cell/ml) with Cyt c peptide and APCs for 24 h. (D) Total CD4+ T cells from normal B10.BR mice were stimulated with immobilized anti– CD3 mAb (5 μg/ml) and soluble anti–CD28 (1 μg/ml) in the presence of medium (−), IL-6 (100 ng/ml), or IL-4 (103 U/ml) and, after 4 d, they were restimulated with immobilized anti–CD3 mAb. (E) Total CD4+ T cells from normal B10.BR mice were stimulated with Con A and APCs in the presence of medium (−), IL-6 (100 ng/ml) (IL-6), or IL-6 (100 ng/ml) plus anti–IL-4 mAb (10 μg/ml) (IL-6 + anti–IL-4), for 4 d. Cells were then restimulated (106 cells/ml) with Con A alone as described in A.
Figure 1
IL-6 directs differentiation of CD4+ T cells into Th2 cells. (A) Total CD4+ T cells (106/ml) were purified from normal B10.BR mice and stimulated (in the presence of 5 × 105 cell/ml syngeneic APCs) with Con A (2.5 μg/ml) alone, Con A plus IL-4 (103 U/ml) or Con A plus IL-12 (3.5 ng/ml), in the absence (−) or presence of IL-6 (100 ng/ml) or IL-2 (50 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cells/ml) with Con A alone (2.5 μg/ml) in the absence of APCs or additional cytokines. Supernatants were harvested 24 h later and analyzed for IL-4 and IFN-γ production by ELISA. (B) Total CD4+ population was isolated from Cyt c TCR transgenic mice, and the naive CD4+ CD45RBhighCD44low subpopulation was purified by cell sorting. Naive CD4+ T cells were then stimulated with Con A alone, Con A plus IL-4, or Con A plus IL-12, and APCs, in the presence or absence of IL-6. After 4 d, cells were exhaustively washed and restimulated (106 cell/ ml) with Con A alone for 24 h before harvesting the supernatants for cytokine measurement. (C) Naive CD4+CD45RBhighCD44low CD4+ T cells isolated from Cyt c TCR transgenic mice were stimulated with Cyt c peptide and APCs, in the presence of medium, IL-6 (100 ng/ml), or IL-4, (103 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cell/ml) with Cyt c peptide and APCs for 24 h. (D) Total CD4+ T cells from normal B10.BR mice were stimulated with immobilized anti– CD3 mAb (5 μg/ml) and soluble anti–CD28 (1 μg/ml) in the presence of medium (−), IL-6 (100 ng/ml), or IL-4 (103 U/ml) and, after 4 d, they were restimulated with immobilized anti–CD3 mAb. (E) Total CD4+ T cells from normal B10.BR mice were stimulated with Con A and APCs in the presence of medium (−), IL-6 (100 ng/ml) (IL-6), or IL-6 (100 ng/ml) plus anti–IL-4 mAb (10 μg/ml) (IL-6 + anti–IL-4), for 4 d. Cells were then restimulated (106 cells/ml) with Con A alone as described in A.
Figure 1
IL-6 directs differentiation of CD4+ T cells into Th2 cells. (A) Total CD4+ T cells (106/ml) were purified from normal B10.BR mice and stimulated (in the presence of 5 × 105 cell/ml syngeneic APCs) with Con A (2.5 μg/ml) alone, Con A plus IL-4 (103 U/ml) or Con A plus IL-12 (3.5 ng/ml), in the absence (−) or presence of IL-6 (100 ng/ml) or IL-2 (50 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cells/ml) with Con A alone (2.5 μg/ml) in the absence of APCs or additional cytokines. Supernatants were harvested 24 h later and analyzed for IL-4 and IFN-γ production by ELISA. (B) Total CD4+ population was isolated from Cyt c TCR transgenic mice, and the naive CD4+ CD45RBhighCD44low subpopulation was purified by cell sorting. Naive CD4+ T cells were then stimulated with Con A alone, Con A plus IL-4, or Con A plus IL-12, and APCs, in the presence or absence of IL-6. After 4 d, cells were exhaustively washed and restimulated (106 cell/ ml) with Con A alone for 24 h before harvesting the supernatants for cytokine measurement. (C) Naive CD4+CD45RBhighCD44low CD4+ T cells isolated from Cyt c TCR transgenic mice were stimulated with Cyt c peptide and APCs, in the presence of medium, IL-6 (100 ng/ml), or IL-4, (103 U/ml). After 4 d, cells were exhaustively washed and restimulated (106 cell/ml) with Cyt c peptide and APCs for 24 h. (D) Total CD4+ T cells from normal B10.BR mice were stimulated with immobilized anti– CD3 mAb (5 μg/ml) and soluble anti–CD28 (1 μg/ml) in the presence of medium (−), IL-6 (100 ng/ml), or IL-4 (103 U/ml) and, after 4 d, they were restimulated with immobilized anti–CD3 mAb. (E) Total CD4+ T cells from normal B10.BR mice were stimulated with Con A and APCs in the presence of medium (−), IL-6 (100 ng/ml) (IL-6), or IL-6 (100 ng/ml) plus anti–IL-4 mAb (10 μg/ml) (IL-6 + anti–IL-4), for 4 d. Cells were then restimulated (106 cells/ml) with Con A alone as described in A.
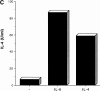
Figure 2
Regulation of IL-6 secretion by APCs during the differentiation of Th1 and Th2 CD4+ T cells. (A) Total CD4+ T cells (B10.BR) were stimulated with Con A (2.5 μg/ml) plus IL-4 (103 U/ml) or IL-12 (3.5 ng/ml) in the presence of APCs. Supernatants were harvested at different times of stimulation (day 2, 3, or 4) and IL-6 secretion was analyzed. (B) Total CD4+ T cells were stimulated as described in A, but in the presence of anti–IL-6Rα chain mAb (10 μg/ml). The arrow indicates the production of IL-6 after 4 d of stimulation with Con A and IL-4, in the absence of anti–IL6Rα mAb. (C) Expression of IL6Rα during the differentiation of Th1 and Th2 cells. CD4+ T cells unstimulated (day 0) or stimulated in the presence of APCs, with Con A plus IL-4 (Con A/IL-4) or IL-12 (Con A/ IL-12) for different periods of time (day 1, 2, or 4) were harvested, stained with anti-CD4 and anti–IL-6Rα mAbs, and analyzed by FACS®. Fluorescence profiles show the expression of IL-6Rα in the CD4+ population.
Figure 2
Regulation of IL-6 secretion by APCs during the differentiation of Th1 and Th2 CD4+ T cells. (A) Total CD4+ T cells (B10.BR) were stimulated with Con A (2.5 μg/ml) plus IL-4 (103 U/ml) or IL-12 (3.5 ng/ml) in the presence of APCs. Supernatants were harvested at different times of stimulation (day 2, 3, or 4) and IL-6 secretion was analyzed. (B) Total CD4+ T cells were stimulated as described in A, but in the presence of anti–IL-6Rα chain mAb (10 μg/ml). The arrow indicates the production of IL-6 after 4 d of stimulation with Con A and IL-4, in the absence of anti–IL6Rα mAb. (C) Expression of IL6Rα during the differentiation of Th1 and Th2 cells. CD4+ T cells unstimulated (day 0) or stimulated in the presence of APCs, with Con A plus IL-4 (Con A/IL-4) or IL-12 (Con A/ IL-12) for different periods of time (day 1, 2, or 4) were harvested, stained with anti-CD4 and anti–IL-6Rα mAbs, and analyzed by FACS®. Fluorescence profiles show the expression of IL-6Rα in the CD4+ population.
Figure 3
IL-6–producing APCs are required for the differentiation of Th2 cells. (A) Total CD4+ T cells (106/ml) were isolated from WT mice and stimulated with Con A (2.5 μg/ml) alone in the presence of APCs (5 × 105 cells/ml) from WT (WT APC) or IL-6–deficient (IL-6− /− APC) mice. After 4 d, CD4+ T cells were washed and restimulated (106 cells/ml) with Con A in the absence of APCs, and supernatants were collected after 24 h. (B) Total CD4+ T cells were isolated from IL-6–deficient (IL-6− /−) mice and stimulated with Con A plus medium (−), neutralizing anti–IL-6 mAb (10 μg/ml) (anti–IL-6), IL-6 (100 ng/ml), IL-4 (103 U/ml), or both (IL-4 + IL-6), in the presence of APCs from WT (WT APC) or IL-6– deficient (IL-6− /−) mice. After 4 d, cells were washed and restimulated (106 cells/ml) with Con A alone for 24 h. Only in the case where all cells (both CD4 T cells and APCs) were from IL-6−/− mice did we observe a slightly lower recovery (70–80% of the recovery from other conditions) after 4 d of primary culture. Nevertheless, cell number was normalized (106 cells/ml) before restimulation. (C) Induction of IL-4 gene expression in CD4+ Th2 cells differentiated in the presence of IL-6. Naive CD4+ T cells (12) from Cyt c TCR transgenic mice were primary cultured with moth Cyt c peptide (5 μg/ml) and APCs in the absence (−) or the presence of IL-6 (100 ng/ml) or IL-4 (103 U/ml) for 4 d. Cells were then washed and stimulated with Cyt c peptide and APCs. After 20 h, 2 × 105 cells were harvested and used for the quantitation of IL-4 mRNA by competitive RT-PCR. (Top) The expression of IL-4 transcripts. (Bottom) The expression of DR transcripts. Small arrow, the competitor DNA.
Figure 3
IL-6–producing APCs are required for the differentiation of Th2 cells. (A) Total CD4+ T cells (106/ml) were isolated from WT mice and stimulated with Con A (2.5 μg/ml) alone in the presence of APCs (5 × 105 cells/ml) from WT (WT APC) or IL-6–deficient (IL-6− /− APC) mice. After 4 d, CD4+ T cells were washed and restimulated (106 cells/ml) with Con A in the absence of APCs, and supernatants were collected after 24 h. (B) Total CD4+ T cells were isolated from IL-6–deficient (IL-6− /−) mice and stimulated with Con A plus medium (−), neutralizing anti–IL-6 mAb (10 μg/ml) (anti–IL-6), IL-6 (100 ng/ml), IL-4 (103 U/ml), or both (IL-4 + IL-6), in the presence of APCs from WT (WT APC) or IL-6– deficient (IL-6− /−) mice. After 4 d, cells were washed and restimulated (106 cells/ml) with Con A alone for 24 h. Only in the case where all cells (both CD4 T cells and APCs) were from IL-6−/− mice did we observe a slightly lower recovery (70–80% of the recovery from other conditions) after 4 d of primary culture. Nevertheless, cell number was normalized (106 cells/ml) before restimulation. (C) Induction of IL-4 gene expression in CD4+ Th2 cells differentiated in the presence of IL-6. Naive CD4+ T cells (12) from Cyt c TCR transgenic mice were primary cultured with moth Cyt c peptide (5 μg/ml) and APCs in the absence (−) or the presence of IL-6 (100 ng/ml) or IL-4 (103 U/ml) for 4 d. Cells were then washed and stimulated with Cyt c peptide and APCs. After 20 h, 2 × 105 cells were harvested and used for the quantitation of IL-4 mRNA by competitive RT-PCR. (Top) The expression of IL-4 transcripts. (Bottom) The expression of DR transcripts. Small arrow, the competitor DNA.
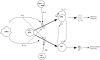
Figure 4
Schematic model for the differentiation of precursors Th cells (pTh) into effector Th1 and Th2 cells.
Similar articles
- Diversion of CD4+ T cell development from regulatory T helper to effector T helper cells alters the contact hypersensitivity response.
DiIulio NA, Xu H, Fairchild RL. DiIulio NA, et al. Eur J Immunol. 1996 Nov;26(11):2606-12. doi: 10.1002/eji.1830261111. Eur J Immunol. 1996. PMID: 8921946 - Co-development of naive CD4+ cells towards T helper type 1 or T helper type 2 cells induced by a combination of IL-12 and IL-4.
Palm N, Germann T, Goedert S, Hoehn P, Koelsch S, Rüde E, Schmitt E. Palm N, et al. Immunobiology. 1996-1997;196(5):475-84. doi: 10.1016/s0171-2985(97)80064-2. Immunobiology. 1996. PMID: 9145325 - The two faces of IL-6 on Th1/Th2 differentiation.
Diehl S, Rincón M. Diehl S, et al. Mol Immunol. 2002 Dec;39(9):531-6. doi: 10.1016/s0161-5890(02)00210-9. Mol Immunol. 2002. PMID: 12431386 Review. - Type 1 T helper and type 2 T helper cells: functions, regulation and role in protection and disease.
Romagnani S. Romagnani S. Int J Clin Lab Res. 1991;21(2):152-8. doi: 10.1007/BF02591635. Int J Clin Lab Res. 1991. PMID: 1687725 Review.
Cited by
- IL-6 deficiency accelerates cerebral cryptococcosis and alters glial cell responses.
Reguera-Gomez M, Munzen ME, Hamed MF, Charles-Niño CL, Martinez LR. Reguera-Gomez M, et al. J Neuroinflammation. 2024 Sep 27;21(1):242. doi: 10.1186/s12974-024-03237-x. J Neuroinflammation. 2024. PMID: 39334365 Free PMC article. - Pretransplant, Th17 dominant alloreactivity in highly sensitized kidney transplant candidates.
Negi S, Rutman AK, Saw CL, Paraskevas S, Tchervenkov J. Negi S, et al. Front Transplant. 2024 Apr 8;3:1336563. doi: 10.3389/frtra.2024.1336563. eCollection 2024. Front Transplant. 2024. PMID: 38993777 Free PMC article. - Mycobacterium tuberculosis and host interactions in the manifestation of tuberculosis.
Abbasnia S, Hashem Asnaashari AM, Sharebiani H, Soleimanpour S, Mosavat A, Rezaee SA. Abbasnia S, et al. J Clin Tuberc Other Mycobact Dis. 2024 Jun 14;36:100458. doi: 10.1016/j.jctube.2024.100458. eCollection 2024 Aug. J Clin Tuberc Other Mycobact Dis. 2024. PMID: 38983441 Free PMC article. Review. - Targeting MELK improves PD-1 blockade efficiency in cervical cancer via enhancing antitumor immunity.
Wang D, Zou F, Li Y, Hu J, Gao L. Wang D, et al. Mol Ther Oncol. 2024 Jan 10;32(1):200759. doi: 10.1016/j.omton.2024.200759. eCollection 2024 Mar 21. Mol Ther Oncol. 2024. PMID: 38596298 Free PMC article. - Glycoprotein 5-Derived Peptides Induce a Protective T-Cell Response in Swine against the Porcine Reproductive and Respiratory Syndrome Virus.
Calderon-Rico F, Bravo-Patiño A, Mendieta I, Perez-Duran F, Zamora-Aviles AG, Franco-Correa LE, Ortega-Flores R, Hernandez-Morales I, Nuñez-Anita RE. Calderon-Rico F, et al. Viruses. 2023 Dec 21;16(1):14. doi: 10.3390/v16010014. Viruses. 2023. PMID: 38275949 Free PMC article.
References
- Paul WE, Seder RA. Lymphocyte response and cytokines. Cell. 1994;76:241–251. - PubMed
- Bretscher PA, Wei G, Menon JN, BielefeldtOhmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major . Science (Wash DC) 1992;257:539–542. - PubMed
- Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials