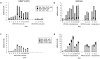Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production - PubMed (original) (raw)
Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production
S A Luther et al. J Exp Med. 1997.
Abstract
Mouse mammary tumor virus (MMTV[SW]) encodes a superantigen expressed by infected B cells. It evokes an antibody response specific for viral envelope protein, indicating selective activation of antigen-specific B cells. The response to MMTV(SW) in draining lymph nodes was compared with the response to haptenated chicken gamma globulin (NP-CGG) using flow cytometry and immunohistology. T cell priming occurs in both responses, with T cells proliferating in association with interdigitating dendritic cells in the T zone. T cell proliferation continues in the presence of B cells in the outer T zone, and B blasts then undergo exponential growth and differentiation into plasma cells in the medullary cords. Germinal centers develop in both responses, but those induced by MMTV(SW) appear later and are smaller. Most T cells activated in the T zone and germinal centers in the MMTV(SW) response are superantigen specific and these persist for weeks in lymph nodes draining the site MMTV(SW) injection: this contrasts with the selective loss of superantigen-specific T cells from other secondary lymphoid tissues. The results indicate that this viral superantigen, when expressed by professional antigen-presenting cells, drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production.
Figures
Figure 1
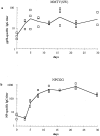
Kinetics of antigen-specific serum IgG antibody responses following immunization with MMTV(SW) or NP-CGG into both footpads. (a) shows the titers against bacterially derived recombinant viral envelope protein gp52 of mice injected with MMTV(SW), and (b) shows the anti-NP titers in mice immunized with alum-precipitated NP-CGG plus B. pertussis. Shown are the individual values (squares) as well as the mean (line).
Figure 2
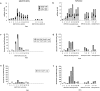
Flow cytometric analysis of T and B cell numbers and proliferative responses. BALB/c mice were immunized into both footpads with MMTV (SW) (a, c, e), or alum-precipitated NP-CGG with B. pertussis (b, d, f). Cell suspensions of the draining popliteal LN were analyzed at different times after immunization. a and b show the mean numbers of B220+ cells, CD4+ cells, and CD8+ cells, per popliteal LN. c and d show the mean numbers of CD4+, Vβ6+, and CD4+, Vβ6− cells that had incorporated BrdU during the 2.5 h before the nodes were removed. e and f show the mean number of CD4− cells that had incorporated BrdU. Extrapolation from histology suggests that the CD4− blasts are mainly B blasts. a also shows results for BALB.D2 mice immunized with MMTV(SW) while b, d, f also show the results for BALB/c mice immunized with alum-precipitated NP-CGG without B. pertussis or mice given proteinfree alum precipitate plus B. pertussis. Three mice were analyzed for each immunization at each timepoint.
Figure 4
Quantitative analysis of T and B cell subsets within the draining popliteal LN responding to MMTV(SW) (a, c), or alum-precipitated NP-CGG plus B. pertussis (b, d). Cell suspensions were analyzed by flow cytometry as in Fig. 2. Values in a and b represent mean numbers of cells/node in different CD4+, Vβ subsets and those in c and d mean numbers of CD4−CD8− cells/node sorted on the basis of their expression of IgD and B220. The same control responses were included as for Fig. 2. Three mice were analyzed for each immunization at each timepoint.
Figure 3
Histological localization of cells proliferating within the T zone (T) of lymph nodes draining MMTV(SW) infection. Cells incorporating BrdU during a 2.5-h pulse are identified by red staining nuclei in all panels. a (×100) shows MHC class II expression (blue) 2 d after challenge with MMTV(SW); large interdigitating cells are seen in the T zone where very few cells are proliferating. The smaller class II+ cells at the edge of the panel are follicular (F) B cells. b–d (×500) show the T zone from a lymph node taken 3 d after infection with MMTV(SW). At this stage there are large numbers of BrdU+ cells in that area. These are associated with IDC stained blue for MHC class II expression in b; they are not obviously associated with B cells stained blue for B220 expression in c and they are largely Vβ6+ as indicated by blue staining in d where recirculating IgD+ B cells are stained brown.
Figure 5
Photomicrographs showing extrafollicular B cell proliferation in response to MMTV(SW). Draining LN were taken 5 d after infection with MMTV(SW) and stained for IgD (brown), BrdU incorporation (red), and in blue either syndecan-1 in a (×250) or Vβ6 in b (×500). Syndecan-1+ plasmablasts are largely seen proliferating within the medullary cords (M). At this stage, Vβ6+ T cells comprise ∼30% of T cells and are almost all located in the T zone (T) where some are in cell cycle. Very few Vβ6+ cells are in the medulla and some of those that are there are likely to be in the efferent lymphatic channels rather than the medullary cords.
Figure 6
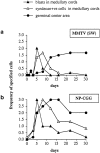
Semiquantitative histological analysis of B cell proliferation and differentiation within the draining LN following immunization with (a) MMTV(SW) or (b) NP-CGG. Slides from the histological study were assessed by two people independently as the extent of reaction in arbitrary units (zero = the level seen in nonimmunized mice, and 3 = a maximum response) and the scores for three mice in each group at each timepoint are averaged. Plasma cells and plasmablasts were identified as being syndecan-1+; blasts are defined as BrdU+ cells. Germinal centers were identified as PNA+B220+IgD− areas.
Figure 7
Photomicrographs of stained sections to show germinal centers formed during responses to NP-CGG in a and MMTV(SW) in b–d within the draining LN. a (×250) shows a LN taken 8 d after immunization with NP-CGG stained for IgD (brown), CD3 (blue) and BrdU (red) incorporation. Proliferating T cells can be seen both within the IgD− germinal center (G) and the surrounding IgD+ follicular mantle (FM). b–d (×160) are serial sections from a draining node 22 d after infection with MMTV(SW) stained for IgD (brown), BrdU (red) and blue staining which shows PNA in b, Vβ6 in c, and CD3 in d, respectively. Almost all the T cells within the PNA+ germinal center (G) and around 10% of the T cells in the T zone (T) express Vβ6. Very few follicular Vβ6 T cells are BrdU+; this applied to T cells in follicles at all stages of the response to MMTV(SW).
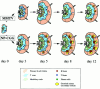
Figure 8
Summary of the major events of the lymph node immune response to either MMTV(SW) superantigen or to NPCGG. This sketch summarizes the responses that were quantified by flow cytometry (Figs. 2 and 4) and localized by immunocytochemistry. (Figs. 3, 5–7). Emphasis is put on the sites of T and B cell activation and differentiation with symbols representing the relative number of cells in a given compartment.
Similar articles
- Early neutralizing antibody response against mouse mammary tumor virus: critical role of viral infection and superantigen-reactive T cells.
Luther SA, Maillard I, Luthi F, Scarpellino L, Diggelmann H, Acha-Orbea H. Luther SA, et al. J Immunol. 1997 Sep 15;159(6):2807-14. J Immunol. 1997. PMID: 9300703 - Recipient polyclonal B cell activation and immunoglobulin production induced by priming with a retroviral superantigen.
Modlin CS, Muruve NA, Stanko D, Caulfield MJ, Fairchild RL. Modlin CS, et al. Cell Immunol. 1996 May 1;169(2):252-63. doi: 10.1006/cimm.1996.0116. Cell Immunol. 1996. PMID: 8620553 - In vivo T cell response to viral superantigen. Selective migration rather than proliferation.
Le Bon A, Lucas B, Vasseur F, Penit C, Papiernik M. Le Bon A, et al. J Immunol. 1996 Jun 15;156(12):4602-8. J Immunol. 1996. PMID: 8648102 - The role of superantigens in the immunobiology of retroviruses.
Huber BT, Beutner U, Subramanyam M. Huber BT, et al. Ciba Found Symp. 1994;187:132-40; discussion 140-3. doi: 10.1002/9780470514672.ch9. Ciba Found Symp. 1994. PMID: 7796668 Review. - Superantigens of mouse mammary tumor virus.
Acha-Orbea H, MacDonald HR. Acha-Orbea H, et al. Annu Rev Immunol. 1995;13:459-86. doi: 10.1146/annurev.iy.13.040195.002331. Annu Rev Immunol. 1995. PMID: 7612231 Review.
Cited by
- Regulation of Th1 and Th2 immune responses by chemokines.
Yoneyama H, Kawasaki S, Matsushima K. Yoneyama H, et al. Springer Semin Immunopathol. 2000;22(4):329-44. doi: 10.1007/s002810000050. Springer Semin Immunopathol. 2000. PMID: 11155440 Review. No abstract available. - The evolution within us.
Cobey S, Wilson P, Matsen FA 4th. Cobey S, et al. Philos Trans R Soc Lond B Biol Sci. 2015 Sep 5;370(1676):20140235. doi: 10.1098/rstb.2014.0235. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26194749 Free PMC article. Review. - B Cell Activation and Plasma Cell Differentiation Are Promoted by IFN-λ in Systemic Lupus Erythematosus.
Barnas JL, Albrecht J, Meednu N, Alzamareh DF, Baker C, McDavid A, Looney RJ, Anolik JH. Barnas JL, et al. J Immunol. 2021 Dec 1;207(11):2660-2672. doi: 10.4049/jimmunol.2100339. Epub 2021 Oct 27. J Immunol. 2021. PMID: 34706932 Free PMC article. - A coordinated change in chemokine responsiveness guides plasma cell movements.
Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. Hargreaves DC, et al. J Exp Med. 2001 Jul 2;194(1):45-56. doi: 10.1084/jem.194.1.45. J Exp Med. 2001. PMID: 11435471 Free PMC article. - Loss of CD154 impairs the Th2 extrafollicular plasma cell response but not early T cell proliferation and interleukin-4 induction.
Cunningham AF, Serre K, Mohr E, Khan M, Toellner KM. Cunningham AF, et al. Immunology. 2004 Oct;113(2):187-93. doi: 10.1111/j.1365-2567.2004.01951.x. Immunology. 2004. PMID: 15379979 Free PMC article.
References
- Acha-Orbea H, MacDonald HR. Superantigens of mouse mammary tumor virus. Annu Rev Immunol. 1995;13:459–486. - PubMed
- Held W, Waanders GA, Shakhov AN, Scarpellino L, Acha-Orbea H, MacDonald HR. Superantigeninduced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993;74:529–540. - PubMed
- Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. - PubMed
- Kawabe Y, Ochi A. Programmed cell-death and extrathymic reduction of Vβ8+ CD4+T cells in mice tolerant to staphylococcus aureus enterotoxin B. Nature (Lond) 1991;349:245–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous