Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus - PubMed (original) (raw)
Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus
B Sodeik et al. J Cell Biol. 1997.
Abstract
Herpes simplex virus 1 fuses with the plasma membrane of a host cell, and the incoming capsids are efficiently and rapidly transported across the cytosol to the nuclear pore complexes, where the viral DNA genomes are released into the nucleoplasm. Using biochemical assays, immunofluorescence, and immunoelectron microscopy in the presence and absence of microtubule depolymerizing agents, it was shown that the cytosolic capsid transport in Vero cells was mediated by microtubules. Antibody labeling revealed the attachment of dynein, a minus end-directed, microtubule-dependent motor, to the viral capsids. We propose that the incoming capsids bind to microtubules and use dynein to propel them from the cell periphery to the nucleus.
Figures
Figure 5
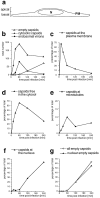
Time course of HSV-1 entry. Vero cells infected at an MOI of 500 in the presence of CH were fixed at various times postinfection with 2% glutaraldehyde in PBS and embedded in Epon for EM analysis. (a) Schematic cross-section perpendicular to the substrate through an adherent Vero cell. For quantitation, 25 random electron micrographs obtained from sections cut parallel to the substrate through the basal part of the cells were taken in a systematic fashion for each time point. “Basal” is defined as being within 2 μm distance from the substrate (30 sections of ∼50–70 nm). (b) The total numbers of cytosolic capsids (full and empty), cytosolic empty capsids, and virions in endosomes were determined for each time point. The number of cytosolic capsids increased rapidly until 1 h postinfection. At later time points, less cytosolic capsids were detected, suggesting that they were ultimately disassembled. In addition, the subcellular localization of each capsid was determined and the numbers were expressed as a percentage of the total (c–g). (c) At the earliest time point of 15 min, the majority of cytosolic capsids was localized close to the plasma membrane, from where they disappeared rapidly later. (d) Several capsids could not be localized to a particular organelle. At 1 h postinfection, there was the highest amount of those capsids, which were apparently free in the cytosol. (e) A significant portion of all cytosolic capsids, namely 10% at 1 h postinfection, colocalized transiently with microtubules. (f) Capsids began to appear at the nuclear pores at 2 h postinfection, and at 4 h, 60% of all cytosolic capsid had reached the nucleus. (g) The appearance of empty cytosolic capsids coincided with the arrival at the nucleus. Only at 4 h postinfection, there was a very small portion of empty, cytosolic capsids. N, nucleus; PM, plasma membrane.
Figure 7
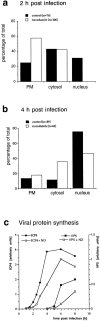
Quantitation of capsid transport and viral protein synthesis in the absence of MTs. Vero cells were infected at an MOI of 170 in the presence of CH and in the absence or presence of nocodazole to depolymerize microtubules. They were fixed at 2 (a) or 4 (b) h postinfection with 1% GA and collected by scraping and pelleting, and Epon sections were prepared. For quantitation, 50 random electron micrographs were obtained in a systematic fashion for each time point and both treatments. The subcellular localization of each cytosolic capsid was determined. (a) At 2 h postinfection in the presence of microtubules, the majority of cytosolic capsids was localized in the cytosol, and a significant fraction had reached the nucleus. In the absence of microtubules, the majority of capsids remained at the plasma membrane (PM), some were in the cytosol, and none had reached the nucleus. (b) At 4 h, >70% of the capsids had reached the nucleus. In the presence of nocodazole, a much smaller fraction was transported to the nucleus, and more capsids remained at the plasma membrane and in the cytosol. (c) Immunoblot. Vero cells were infected at an MOI of 0.5 (without CH!) in the absence or presence of 5 μM nocodazole for various times. The cells were harvested and lysed in 0.5% TX-100 in buffer, the nuclei were pelleted, and the supernatants were analyzed by immunoblotting. To monitor viral protein synthesis, antibodies to ICP4, an early viral protein, and to VP5, a late viral protein, were used. Calnexin, a membrane-bound ER protein, was used as a cellular marker protein. Equal amounts of protein were loaded per lane, and the amount of ICP4 or VP5 was normalized with the amount of calnexin. ICP4 synthesis commences at 2 h PI, whereas VP5 is first detectable at 5 h PI. The amount of ICP4 is maximal after 6 h PI. In contrast, VP5 synthesis increases during the whole time course of the experiment. At all time points, lower amounts of both, ICP4 and VP5, are synthesized in the absence of microtubules.
Figure 1
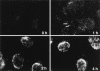
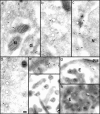
Entry and uncoating of HSV-1. Ultrathin Epon sections of Vero cells infected with HSV-1 at an MOI of 500 in the presence of cycloheximide (CH). After 2 h of virus binding at 4°C, the cells were either fixed immediately (a and b), or warmed up for 30 (c–f) or 60 min (g and h). (a and b) Binding. The main morphological features of the intact virus bound to the plasma membrane (PM) are the viral envelope (b, arrowhead) with the viral spikes (a, arrowhead), the viral capsid (arrow), and the electron-dense viral DNA genome within the capsid. (c and d) Fusion. Upon warming up, the viral envelope fuses with the plasma membrane, and the capsid (arrow) and the tegument proteins are released into the cytosol. (e and f) Release of the capsid. Preparations, which display a prominent contrast of the tegument, show that the tegument (arrowheads) stays behind at the PM. (g and h) Binding to the nuclear pore. At later time points, the capsids (arrows) have arrived at the nuclear envelope (NE), where they are exclusively located in close apposition to the nuclear pore complexes. Occasionally, fibers emanating from the pores (arrowheads) are visible, to which the capsids seem to bind. Almost all of the capsids at the nuclear pores appear empty and have lost the electron-dense DNA core. Bar, 100 nm.
Figure 2
Efficiency and kinetics of HSV-1 binding and internalization. (a) Using [3H]thymidine-labeled virus at different multiplicities of infection (MOI), the amount of virus bound to cells at 4°C was quantified after various time points. There is no apparent saturation of virus binding to Vero cells. After 2 h, ∼40% of the virus added bound. (b) Using [3H]thymidine-labeled virus at different MOIs, the amount of virus internalized into cells was quantified after various time points. At time 0 min (equals 2 h of virus binding at 4°C), proteinase K treatment removes >95% of the virus bound to the plasma membrane. Upon warming up to 37°C and viral fusion, the virus is protected within the cells from the protease treatment. After 30 min, the maximal amount of virus, ∼70% of the bound virus, is internalized. The half-time (t1/2) of internalization is 8 min. Depolymerization of microtubules by nocodazole (ND) has no effect on the kinetics and the amount of internalization.
Figure 3
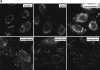
Subcellular distribution of incoming HSV-1 capsids. Conventional immunofluorescence microscopy of Vero cells infected with HSV-1 at an MOI of 50 in the presence of CH. The cells were fixed in 3% PFA, permeabilized with 0.1% TX-100, and labeled with anti-HC, an antibody generated against purified capsids. After 2 h of virus binding at 4°C (0 h), there is no signal. At 1 h PI, numerous cytoplasmic capsids (arrows) distributed within the entire cytoplasm are detectable. At 2 h PI, the capsids begin to accumulate at the nucleus. At 4 h PI, most of the capsids decorate the nuclei. Note that at 1 h, the focus was close to the coverslip, whereas at 2 and 4 h, it was at the nuclear membrane.
Figure 4
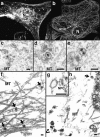
Incoming HSV-1 capsids colocalize with MTs. (a) Conventional immunofluorescence microscopy. Vero cells infected at an MOI of 50 in the presence of CH were fixed in methanol at 2 h PI, and double labeled with anti-VP5 (NC-1; FITC anti–rabbit) and antitubulin antibodies (IA2; rhodamine anti–mouse). The FITC and the rhodamine signals were documented simultaneously using the FITC filter set. Almost all capsids (arrows) not localized to the nucleus (N) colocalize with microtubules. (b) Confocal immunofluorescence microscopy. Vero cells infected at an MOI of 50 in the presence of CH were fixed in methanol 2 h PI, and double labeled with anti-capsid (HC, white) and anti-tubulin antibodies (IA2, gray). Almost all capsids not localized to the nucleus (N) colocalize with microtubules. Note that in some cells, the viral capsids accumulate at the microtubule-organizing center (MTOC). (c–e) Conventional EM. At 1 h postinfection, Vero cells infected at an MOI of 500 in the presence of CH were fixed with 2% glutaraldehyde in PBS. Epon sections were cut parallel to the substrate. Numerous incoming viral capsids are localized to microtubules (MT) as identified by their typical morphology, localization, and their 24-nm diameter. (f–h) Immunoelectron microscopy. Vero cells infected at an MOI of 500 in the presence of CH were extracted at 1 h (f) or 4 h PI (g and h) with 0.5% TX-100 in MT buffer before fixation. They were labeled with rabbit anti– tubulin followed by protein A–9 nm gold, and Epon sections were cut parallel to the substrate. The cytoskeleton, nucleus, and nuclear pore complexes (NPC), as well as cytoplasmic capsids, were preserved excellently, whereas all membranous organelles were extracted. The microtubules (MT) are easily identified, since they are heavily decorated with antibody and protein A–gold. In rather thick sections (f), the microtubules can be traced over long distances, and many capsids (filled arrows) are localized on them. These capsids still contain their electron-dense DNA core. At later time points (g and h), most of the capsids have lost their electron-dense core (h, open arrow) and are mostly located in close proximity to the nucleus. Very often the capsids are bound directly to the outer ring of the nuclear pore complex (g, NPC). Virions bound to the plasma membrane (h, curved arrowhead) have lost the trilaminar appearance of their membrane, but apparently a lot of glycoprotein and tegument remains attached to them, causing a distinct morphology easily distinguished from cytoplasmic capsids (g). Bar, 100 nm.
Figure 6
Reduced transport of capsids to the nucleus in the absence of MTs. Immunofluorescence microscopy of Vero cells infected with HSV-1 for 2 h at an MOI of 50 in the presence of CH and drugs affecting the cytoskeleton. (a) Cytoskeletal drugs. Under control conditions, almost all capsids accumulate around the nucleus. If the cells were infected in the presence of nocodazole, colchicine, or vinblastine, respectively (lower panel), there is no accumulation of viral capsids at the nucleus; instead, the capsids are scattered throughout the entire cytoplasm. In cells infected in the presence of taxol or cytochalasin D, the capsids accumulate at the nucleus (upper panel). The cells were fixed in 3% PFA, permeabilized with 0.1% TX-100, and labeled with an antibody directed against purified capsids (HC). (b) Reversibility. In the presence of nocodazole, the amount of viral capsids localized to the nucleus is drastically reduced, whereas, after a 2-h chase in nocodazole-free medium, the capsids accumulate around the cell nuclei. Cells were infected in the presence of nocodazole for 2 h, fixed immediately (left panels), or washed and further incubated in normal medium for an additional 2 h before fixation (right panels). After extraction with 0.5% TX-100 in MT buffer and fixation in methanol, the cells were double labeled with anti-tubulin (left panels) and anti-capsid (HC, right panels).
Figure 6
Reduced transport of capsids to the nucleus in the absence of MTs. Immunofluorescence microscopy of Vero cells infected with HSV-1 for 2 h at an MOI of 50 in the presence of CH and drugs affecting the cytoskeleton. (a) Cytoskeletal drugs. Under control conditions, almost all capsids accumulate around the nucleus. If the cells were infected in the presence of nocodazole, colchicine, or vinblastine, respectively (lower panel), there is no accumulation of viral capsids at the nucleus; instead, the capsids are scattered throughout the entire cytoplasm. In cells infected in the presence of taxol or cytochalasin D, the capsids accumulate at the nucleus (upper panel). The cells were fixed in 3% PFA, permeabilized with 0.1% TX-100, and labeled with an antibody directed against purified capsids (HC). (b) Reversibility. In the presence of nocodazole, the amount of viral capsids localized to the nucleus is drastically reduced, whereas, after a 2-h chase in nocodazole-free medium, the capsids accumulate around the cell nuclei. Cells were infected in the presence of nocodazole for 2 h, fixed immediately (left panels), or washed and further incubated in normal medium for an additional 2 h before fixation (right panels). After extraction with 0.5% TX-100 in MT buffer and fixation in methanol, the cells were double labeled with anti-tubulin (left panels) and anti-capsid (HC, right panels).
Figure 8
Incoming HSV-1 capsids display appendices at their vertices. At 1 h PI, Vero cells infected at an MOI of 500 in the presence of CH were extracted with 0.5% TX-100 in MT buffer for 2 min at 37°C and fixed in 1% GA in MT buffer. They were labeled with anti-tubulin followed by protein A–9 nm gold (a) or embedded directly (b–d), and Epon sections were cut parallel to the substrate. All viral capsids display some appendages on their outside (arrowheads). The appendages are visible at three, four, five, or all six corners visible in a section, which represent the pentons of a viral capsid. Some of the appendices have a morphology very similar to purified cytoplasmic dynein (large arrow in d): a large globular stalk with two smaller globular head domains. The capsid size is ∼100–110 nm; the dynein-like appendices are ∼50 nm. In thick sections of golden color, the appendices are often buried in cytoskeletal material (b and d), whereas in very thin sections of grey color (a and c), they are very prominent. The appendices have all kinds of different morphology with varying sizes and electron density, but they are always located at the pentons.
Figure 9
Anti-dynein antibodies label incoming viral HSV-1 capsids. Thawed cryosections of Vero cells infected for 1 (c) or 2 h (a, b, and d–h) in the presence of CH at an MOI of 500 were labeled with rabbit antibodies followed by protein A–5 nm (a–d, and g) or 10 nm gold (e, g, and h). About 5% of the incoming viral capsids (a–f, and h) are labeled by anti-dynein (arrows), an affinity-purified antibody made against a recombinant fragment of dynein heavy. Labeled capsids are localized close to the plasma membrane (g, PM), at the nuclear envelope (c and d), or within the cytoplasm without obvious connection to either one (b). Note that the capsids within the extracellular virions (f) or within the endosomes (g and h, E) are not labeled. Anti-dynein also labels the membranes of mitochondria (a, M), endosomes (h, E), and the Golgi complex (a, G) and diffusely the entire cytoplasm, whereas the mitochondrial matrix (a, b, and d) or the nucleus (d, N) show no labeling for dynein. Bar, 100 nm.
Similar articles
- Exploitation of microtubule cytoskeleton and dynein during parvoviral traffic toward the nucleus.
Suikkanen S, Aaltonen T, Nevalainen M, Välilehto O, Lindholm L, Vuento M, Vihinen-Ranta M. Suikkanen S, et al. J Virol. 2003 Oct;77(19):10270-9. doi: 10.1128/jvi.77.19.10270-10279.2003. J Virol. 2003. PMID: 12970411 Free PMC article. - Eclipse phase of herpes simplex virus type 1 infection: Efficient dynein-mediated capsid transport without the small capsid protein VP26.
Döhner K, Radtke K, Schmidt S, Sodeik B. Döhner K, et al. J Virol. 2006 Aug;80(16):8211-24. doi: 10.1128/JVI.02528-05. J Virol. 2006. PMID: 16873277 Free PMC article. - Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport.
Douglas MW, Diefenbach RJ, Homa FL, Miranda-Saksena M, Rixon FJ, Vittone V, Byth K, Cunningham AL. Douglas MW, et al. J Biol Chem. 2004 Jul 2;279(27):28522-30. doi: 10.1074/jbc.M311671200. Epub 2004 Apr 26. J Biol Chem. 2004. PMID: 15117959 - The journey of herpesvirus capsids and genomes to the host cell nucleus.
Döhner K, Cornelius A, Serrero MC, Sodeik B. Döhner K, et al. Curr Opin Virol. 2021 Oct;50:147-158. doi: 10.1016/j.coviro.2021.08.005. Epub 2021 Aug 28. Curr Opin Virol. 2021. PMID: 34464845 Review. - A Hitchhiker's Guide Through the Cell: The World According to the Capsids of Alphaherpesviruses.
Döhner K, Serrero MC, Viejo-Borbolla A, Sodeik B. Döhner K, et al. Annu Rev Virol. 2024 Sep;11(1):215-238. doi: 10.1146/annurev-virology-100422-022751. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38954634 Review.
Cited by
- The UL36 tegument protein of herpes simplex virus 1 has a composite binding site at the capsid vertices.
Cardone G, Newcomb WW, Cheng N, Wingfield PT, Trus BL, Brown JC, Steven AC. Cardone G, et al. J Virol. 2012 Apr;86(8):4058-64. doi: 10.1128/JVI.00012-12. Epub 2012 Feb 15. J Virol. 2012. PMID: 22345483 Free PMC article. - Dynein-dependent transport of the hantaan virus nucleocapsid protein to the endoplasmic reticulum-Golgi intermediate compartment.
Ramanathan HN, Chung DH, Plane SJ, Sztul E, Chu YK, Guttieri MC, McDowell M, Ali G, Jonsson CB. Ramanathan HN, et al. J Virol. 2007 Aug;81(16):8634-47. doi: 10.1128/JVI.00418-07. Epub 2007 May 30. J Virol. 2007. PMID: 17537852 Free PMC article. - Membrane targeting properties of a herpesvirus tegument protein-retrovirus Gag chimera.
Bowzard JB, Visalli RJ, Wilson CB, Loomis JS, Callahan EM, Courtney RJ, Wills JW. Bowzard JB, et al. J Virol. 2000 Sep;74(18):8692-9. doi: 10.1128/jvi.74.18.8692-8699.2000. J Virol. 2000. PMID: 10954570 Free PMC article. - Visualization of the African swine fever virus infection in living cells by incorporation into the virus particle of green fluorescent protein-p54 membrane protein chimera.
Hernaez B, Escribano JM, Alonso C. Hernaez B, et al. Virology. 2006 Jun 20;350(1):1-14. doi: 10.1016/j.virol.2006.01.021. Epub 2006 Feb 21. Virology. 2006. PMID: 16490226 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources