Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target - PubMed (original) (raw)
Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target
L J Richards et al. J Neurosci. 1997.
Abstract
Projection neurons throughout the mature mammalian neocortex extend efferent axons either through the ventrolaterally positioned internal capsule to subcortical targets or through the dorsally located midline corpus callosum to the contralateral cortex. In rats, the internal capsule is pioneered on E14, but the corpus callosum is not pioneered until E17, even though these two types of projection neurons are generated at the same time. Here we use axonal markers to demonstrate that early cortical axon growth is directed toward the nascent internal capsule, which could account for the timing difference in the development of the two efferent pathways. This directed axon growth may be due to a chemoattractant and/or a chemorepellent secreted by intermediate targets of cortical efferent axons, the nascent internal capsule, or the medial wall of the dorsal telencephalon (MDT), respectively. To test for these soluble activities, explants of E15 rat neocortex and intermediate targets were cocultured in collagen gels. Cortical axon outgrowth was directed toward the internal capsule, but outgrowth was nondirected and suppressed when cocultured with MDT, suggesting that the internal capsule releases a chemoattractant for cortical axons, whereas the MDT releases a chemosuppressant. Because the chemoattractant Netrin-1 is expressed in the internal capsule, we cocultured cortical explants with E13 rat floor plate, which expresses Netrin-1, or with Netrin-1-transfected or control-transfected 293T cells. Cortical axon growth was directed toward both floor plate and Netrin-1-transfected 293T cells, as it had been toward the internal capsule, but not toward control-transfected 293T cells. These findings suggest that early events in cortical axon pathfinding may be controlled by a soluble activity which attracts initial axon growth toward the internal capsule and that this activity may be due to Netrin-1.
Figures
Fig. 1.
Cortical axons extend laterally and medially at E17. Pattern of labeling from a neocortical DiI injection in a rat brain fixed at E17. A–C are serial coronal sections progressing from rostral (R) to caudal (C); dorsal is to the top, lateral to the_left_. One section is not shown between _C_and D. Unlike at earlier ages, at E17, large numbers of retrogradely labeled cells are found both lateral and medial to the injection site (asterisk). Laterally placed cells extend axons medially along the callosal trajectory. CP, Cortical plate; SP, subplate; IZ, intermediate zone; NE, neuroepithelium;LV, lateral ventricle. Scale bar, 250 μm.
Fig. 2.
Developmental series of TuJ1-immunostained coronal sections from brains of rat embryos ages E14–E18. Scale bar, 250 μm. In each photomontage, medial is to the left, dorsal to the top. Stars mark the expected crossing point of the corpus callosum. PP, Preplate. Other abbreviations as in Figure 1.
Fig. 6.
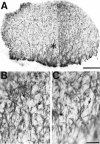
The earliest cortical axon growth is predominantly directed laterally. A, Whole mount of an E12 rat cortical hemisphere immunostained with the neuron-specific marker TuJ1. Rostral is to the left, lateral is down. The asterisk marks the location from which_B_ and C are taken. B, C, Higher magnification images of lateral regions of the brain shown in_A_. Some immunostained cells whose axons are visible are indicated with arrows. Most axons extend laterally toward the internal capsule. Scale bars: A, 250 μm;B, C (shown in C), 50 μm.
Fig. 3.
A dorsal see-through view of a cortical hemisphere summarizing the predominant pattern of cortical axon extension at E14, E15, and E16. Medial is to the left, rostral is to the_top_. The approximate locations of the internal capsule and lateral ventricle underlying the cortical mantle are indicated. Although the most medial axons must take somewhat indirect trajectories, most axon extension at these ages is directed toward the ventrolaterally located internal capsule.
Fig. 4.
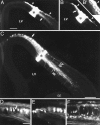
DiI injections into neocortex at E14 through E16 reveal that axon extension is predominantly directed laterally toward the internal capsule. Coronal sections: dorsal to the_top_, lateral to the left. A, B, Adjacent sections through an E14 fixed brain showing the pattern of labeling from a single DiI injection (asterisks). Virtually all retrogradely labeled cells are found medial to the injection site (arrow), and anterogradely labeled axons are found lateral to it (arrowheads). This labeling pattern indicates that most cells extend axons laterally at this stage. The efferent axons extend within the intermediate zone (IZ), deep to the preplate (PP). The superficial-most labeling both medial and lateral to the DiI injection site is nonspecific labeling of the pia attributable to DiI diffusion. B′, Higher power view of the cortical wall medial to the injection site in B. The_arrowhead_ marks nonspecific DiI labeling in the pia. A row of retrogradely labeled cortical cells is marked by the_arrow_. C, Low magnification image showing the pattern of labeling from an injection (asterisk) at E16. Most retrogradely labeled cells were medial to the injection (arrow). D, E, Higher magnification images of cells (arrows) retrogradely labeled by such an injection. F, Retrogradely labeled cells from a more ventrolaterally placed injection. At this level, both subplate (SP) and cortical plate (CP) cells are clearly distinguishable. Both populations extend axons laterally; very few cells of either population extend axons medially at this age. Abbreviations as in Figure 1. Scale bars: A, B (shown in_A_), 250 μm; B′, 100 μm;C, 250 μm; D–F (shown in_D_), 50 μm.
Fig. 5.
DiI injections in frontal and frontomedial cortex in E16 brains. Coronal sections: dorsal to the top, lateral to the left. A, A′, An injection (asterisk) into frontal cortex retrogradely labeled cells at more medial positions as far as 600 μm caudal in presumptive retrosplenial cortex. B, An injection (asterisk) of DiI into medial cortex retrogradely labeled cells (B′) several hundred micrometers caudal in presumptive cingulate cortex. The midline is marked by an_arrowhead. LV_, Lateral ventricle. Scale bar, 150 μm.
Fig. 7.
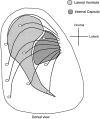
Schematic representation of coculture experiments. A, Dorsal view of an E15 rat brain showing where cortical explants were derived from within the dorsal telencephalon (i.e., neocortex). B, Coronal section through an E15 rat forebrain showing the locations from which explants of the intermediate target explants of cortical axons, the nascent internal capsule and the MDT, were dissected.C, Netrin-1-secreting explants were the floor plate, taken from an E13 rat spinal cord shown in transverse section, and agar-embedded HEK 293T cells transfected with Netrin-1 cDNA in the sense orientation. Agar-embedded HEK 293T cells transfected with Netrin-1 cDNA in the antisense orientation were used as a control.D, Cortical explants were cultured in collagen gels either alone or with test explants of either internal capsule, MDT, floor plate, or small agar cubes containing transfected 293T cells. For analysis of axon outgrowth, cortical explants were divided into four quadrants as shown by the dotted lines. The axon outgrowth from the sides of the cortical explant toward (t) and away (a) from the test explants was analyzed.
Fig. 8.
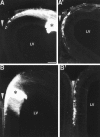
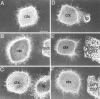
The internal capsule releases a chemoattractant activity for early cortical axons that can be mimicked by a soluble activity released by floor plate and by Netrin-1-transfected 293T cells. Cortical explants (ctx) were cultured in collagen gels either alone (A) or with test explants of MDT (B; MDT), nascent internal capsule (C; ic), floor plate (D;fp), or 293T cells transfected with Netrin-1 cDNA (E; Netrin-1 293T), or 293T cells transfected with a control construct (F; cont. 293T). The cortical explants were placed with their pial surface up, and either their medial or lateral side facing the test explant. A, E15 rat cortical explants cultured alone extended axons in a nondirected manner. B, Similarly, cortical explants cocultured with explants of E15 rat MDT extended axons in a nondirected manner. C, In contrast, cortical explants cocultured with explants of E15 rat internal capsule (ic) showed robust axon outgrowth directed toward the internal capsule explant. D–F, The effect of the internal capsule could be mimicked by coculturing the cortical explants with either floor plate explants (D) or agar cubes containing 293T cells transfected with Netrin-1 (E). In these cocultures, growth toward the floor plate or Netrin-1-transfected 293T cells was robust. In contrast, cortical explants cocultured with agar cubes containing control-transfected 293T cells extended axons in a nondirected manner (F). Scale bar, 200 μm.
Fig. 9.
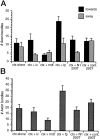
Quantification of axon bundles in each type of cortical explant culture. A, Mean number (±SEM) of axon bundles extended from the cortical explants (ctx) toward (▪) or away (░) from test explants. ic, Internal capsule; fp, floor plate; N1–293T, agar cubes of 293T cells transfected with Netrin-1 cDNA; cont 293T, agar cubes of 293T cells transfected with Netrin-1 cDNA in the antisense orientation. The perimeter surrounding each cortical explant was divided into four quadrants (see Fig. 7), and the total number of axon bundles >150 μm in length, found in either the toward quadrant or the away quadrant, was counted. For cortical explants cultured alone (ctx alone), the toward quadrant was to the right and the away quadrant to the_left_. B, Mean total number (±SEM) of axon bundles extended both toward and away from the test explants (i.e., sum of the data presented in A). The number of cases scored is the same as the total number of explants indicated in Table 1. These parametric data were statistically analyzed using Student’s t test.
Fig. 10.
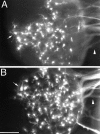
Cortical axons extended toward test explants arise from neurons distributed throughout the cortical explants. DiI injected into the collagen gel to the right side (i.e., toward side) of the cortical explants retrogradely labels neurons distributed across cortical explants cultured alone (A) or cocultured with floor plate (B).Arrowheads point to bundles of retrogradely labeled cortical axons extended into the collagen gel. Arrows_mark retrogradely labeled neurons at the edge of the side of the cortical explants opposite the injection site (i.e., the away side). In_B, the injection was placed between the cortical explant and the floor plate. In each case, only a proportion of the axons extending into the collagen contact the DiI injection site. Scale bar, 200 μm.
Similar articles
- Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection.
Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O'Leary DD. Braisted JE, et al. J Neurosci. 2000 Aug 1;20(15):5792-801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. J Neurosci. 2000. PMID: 10908620 Free PMC article. - A role for netrin-1 in the guidance of cortical efferents.
Métin C, Deléglise D, Serafini T, Kennedy TE, Tessier-Lavigne M. Métin C, et al. Development. 1997 Dec;124(24):5063-74. doi: 10.1242/dev.124.24.5063. Development. 1997. PMID: 9362464 - Axons of early generated neurons in cingulate cortex pioneer the corpus callosum.
Koester SE, O'Leary DD. Koester SE, et al. J Neurosci. 1994 Nov;14(11 Pt 1):6608-20. doi: 10.1523/JNEUROSCI.14-11-06608.1994. J Neurosci. 1994. PMID: 7965064 Free PMC article. - The role of the floor plate in axon guidance.
Colamarino SA, Tessier-Lavigne M. Colamarino SA, et al. Annu Rev Neurosci. 1995;18:497-529. doi: 10.1146/annurev.ne.18.030195.002433. Annu Rev Neurosci. 1995. PMID: 7605072 Review. - Complex patterns and simple architects: molecular guidance cues for developing axonal pathways in the telencephalon.
Judas M, Milosević NJ, Rasin MR, Heffer-Lauc M, Kostović I. Judas M, et al. Prog Mol Subcell Biol. 2003;32:1-32. doi: 10.1007/978-3-642-55557-2_1. Prog Mol Subcell Biol. 2003. PMID: 12827969 Review. No abstract available.
Cited by
- Morphology and growth patterns of developing thalamocortical axons.
Skaliora I, Adams R, Blakemore C. Skaliora I, et al. J Neurosci. 2000 May 15;20(10):3650-62. doi: 10.1523/JNEUROSCI.20-10-03650.2000. J Neurosci. 2000. PMID: 10804207 Free PMC article. - Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain.
López-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marín O. López-Bendito G, et al. J Neurosci. 2007 Mar 28;27(13):3395-407. doi: 10.1523/JNEUROSCI.4605-06.2007. J Neurosci. 2007. PMID: 17392456 Free PMC article. - Development and malformations of the human pyramidal tract.
ten Donkelaar HJ, Lammens M, Wesseling P, Hori A, Keyser A, Rotteveel J. ten Donkelaar HJ, et al. J Neurol. 2004 Dec;251(12):1429-42. doi: 10.1007/s00415-004-0653-3. J Neurol. 2004. PMID: 15645341 Review. - Branch management: mechanisms of axon branching in the developing vertebrate CNS.
Kalil K, Dent EW. Kalil K, et al. Nat Rev Neurosci. 2014 Jan;15(1):7-18. doi: 10.1038/nrn3650. Nat Rev Neurosci. 2014. PMID: 24356070 Free PMC article. Review. - Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance.
Briançon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, Fromont S, Piché C, Enslen H, Chebli K, Cloutier JF, Castellani V, Debant A, Lamarche-Vane N. Briançon-Marjollet A, et al. Mol Cell Biol. 2008 Apr;28(7):2314-23. doi: 10.1128/MCB.00998-07. Epub 2008 Jan 22. Mol Cell Biol. 2008. PMID: 18212043 Free PMC article.
References
- Adams NC, Baker GE. Cells of the perireticular nucleus project to the developing neocortex in the rat. J Comp Neurol. 1995;359:613–626. - PubMed
- Ausebel F, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl K. In: Current protocols in molecular biology (Janssen K, ed), pp 9.1.1–9.1.3. Wiley; New York: 1987.
- Bayer SA, Altman J. Neocortical Development. Raven; New York: 1991.
- Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources