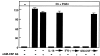Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation - PubMed (original) (raw)
Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation
N Udagawa et al. J Exp Med. 1997.
Free PMC article
Abstract
We have established by differential display polymerase chain reaction of mRNA that interleukin (IL)-18 is expressed by osteoblastic stromal cells. The stromal cell populations used for comparison differed in their ability to promote osteoclast-like multinucleated cell (OCL) formation. mRNA for IL-18 was found to be expressed in greater abundance in lines that were unable to support OCL formation than in supportive cells. Recombinant IL-18 was found to inhibit OCL formation in cocultures of osteoblasts and hemopoietic cells of spleen or bone marrow origin. IL-18 inhibited OCL formation in the presence of osteoclastogenic agents including 1alpha,25-dihydroxyvitamin D3, prostaglandin E2, parathyroid hormone, IL-1, and IL-11. The inhibitory effect of IL-18 was limited to the early phase of the cocultures, which coincides with proliferation of hemopoietic precursors. IL-18 has been reported to induce interferon-gamma (IFN-gamma) and granulocyte/macrophage colony-stimulating factor (GM-CSF) production in T cells, and both agents also inhibit OCL formation in vitro. Neutralizing antibodies to GM-CSF were able to rescue IL-18 inhibition of OCL formation, whereas neutralizing antibodies to IFN-gamma did not. In cocultures with osteoblasts and spleen cells from IFN-gamma receptor type II-deficient mice, IL-18 was found to inhibit OCL formation, indicating that IL-18 acted independently of IFN-gamma production: IFN-gamma had no effect in these cocultures. Additionally, in cocultures in which spleen cells were derived from receptor-deficient mice and osteoblasts were from wild-type mice and vice versa, we identified that the target cells for IFN-gamma inhibition of OCL formation were the hemopoietic cells. The work provides evidence that IL-18 is expressed by osteoblasts and inhibits OCL formation via GM-CSF production and not via IFN-gamma production.
Figures
Figure 1
Identification of IL-18. (A) An example of a ddPCR gel. Lanes correspond to RNA from the different sources: (lane 1) hydrocortisone-treated tsJ10 cells, (lane 2) hydrocortisone-treated tsJ14 cells, (lane 3) 1α,25(OH)2 D3- and PGE2treated tsJ2 cells, and (lane 4) 1α,25(OH)2 D3- and PGE2treated tsJ14 cells. The PCR fragment identified as IL-18 is indicated by the arrow on the left. Indicated by the arrow on the right is a PCR fragment corresponding to a hitherto uncharacterized mRNA species, which is expressed in greater abundance in the OCL-supportive cell lines. The osteoclast-supporting activity (OSA) of these cell lines is indicated below the gel: plus (supportive) or minus (nonsupportive). (B) Nucleotide sequence of mouse IL-18 (GenBankTM accession number D49949). The region corresponding to the differentially expressed PCR fragment isolated from (A) is between nucleotides 636–830. Sequences underlined correspond to oligonucleotides specific to IL-18 used for RT-PCR analysis and detection of RT-PCR products (IL-18-1, IL-18-3, and IL-18-2 from 5′ to 3′). Nucleotides in capitals corrrespond to the coding region of IL-18, whereas those in lower case correspond to the 5′ and 3′ untranslated sequences. (C) Semiquantitative RT-PCR analysis of IL-18 mRNA. PCR products for RNA isolated from different sources was reversed transcribed with oligo (dT) and PCR performed with the primers IL-18-1 and IL-18-2 for 23 cycles, which was in the log-linear range of amplification. Lanes correspond to RNA from (1) hydrocortisone-treated tsJ10 cells, (2) hydrocortisone-treated tsJ14 cells, (3) 1α,25(OH)2 D3- and PGE2-treated tsJ2 cells, and (4) 1α,25(OH)2 D3- and PGE2-treated tsJ14 cells. Resultant PCR products were electrophoresed, transferred to a nylon membrane, and hybridized with a γ-32P–labeled internal detection oligonuleotide for IL-18 (IL-18-3). Similar amplifications for GAPDH with GAPDH-2 and GAPDH-4 for 20 cycles were performed and products detected with γ-32P–labeled GAPDH-1 as previously described (30). The osteoclast-supporting activity (OSA) of these cell lines is indicated below the gel: plus (supportive) or minus (nonsupportive).
Figure 2
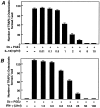
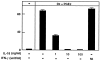
OCL formation in cocultures of mouse bone marrow and osteoblastic cells in the presence of IL-18 (A) or IFN-γ (B). Mouse bone marrow and primary osteoblastic cells were cocultured with 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M) in the presence of increasing concentrations of IL-18 (A) or IFN-γ (B). For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 and PGE2, respectively. After culture for 7 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures, and are representative of three similar experiments.
Figure 3
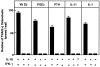
Effect of IL-18 (10 ng/ml) on OCL formation in cocultures of mouse bone marrow and osteoblastic cells in the presence of 1α,25(OH)2 D3 (10−8 M), PGE2 (10−7 M), PTH (200 ng/ml), IL-11 (20 ng/ml), and IL-1 (100 ng/ml). After culture for 7 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures and are representative of three similar experiments.
Figure 4
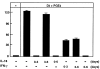
Effect of IL-18 and IFN-γ on OCL formation in cocultures of mouse bone marrow and osteoblastic cells during the coculture period in the absence and presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M). IL-18 (10 ng/ml) and IFN-γ (50 U/ml) were present over the entire culture period (days 0–6) or during the first 3 d (0–3) or the last 3 d (–6). Media change occurred at day 3 of the culture. After culture for 6 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. This experiment was repeated on two further occasions with similar results.
Figure 5
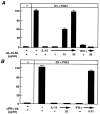
Effect of neutralizing antibodies against IL-18 or IFN-γ in rescuing OCL formation in cocultures of mouse bone marrow and osteoblastic cells treated with IL-18 or IFN-γ. Cocultures were incubated in the presence or absence of IL-18 (10 ng/ml) and IFN-γ (50 U/ml) and the effect of antibodies against IL-18 (A) or IFN-γ (B) were determined. For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M), respectively. After culture for 7 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. This experiment was repeated twice.
Figure 6
OCL formation in cocultures of spleen cells and osteoblastic cells derived from IFN-γ receptor type II knockout (IFN-γ R −/−) mice. IL-18 (10 ng/ml) or IFN-γ (50 U/ml) were present over the entire culture period. For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M), respectively. After culture for 7 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. This experiment was repeated twice.
Figure 7
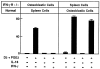
OCL formation in cocultures of normal C57/BL6 mousederived spleen cells with osteoblastic cells derived from IFN-γ R−/− mice and cocultures of normal C57BL/J6 mouse-derived osteoblastic cells with spleen cells derived from IFN-γ R−/− mice. Cocultures were performed in the presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M) and treated with IL-18 (10 ng/ml) or IFN-γ (50 U/ml). For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 and PGE2, respectively. After culture for 7 d, TRAPpositive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. This experiment was repeated twice.
Figure 8
Effect of neutralizing antibodies against GM–CSF to rescue OCL formation in cocultures of mouse bone marrow and osteoblastic cells treated with GM–CSF (0.1 ng/ml), IL-18 (10 ng/ml), or IFN-γ (50 U/ml). Cocultures were incubated in the presence or absence of GM– CSF, IL-18, or IFN-γ and the effect of neutralizing antibodies to GM– CSF (1 μg/ml) were determined. For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M), respectively. After culture for 7 d, TRAPpositive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. Similar results were obtained with three repeat experiments.
Similar articles
- Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor.
Horwood NJ, Udagawa N, Elliott J, Grail D, Okamura H, Kurimoto M, Dunn AR, Martin T, Gillespie MT. Horwood NJ, et al. J Clin Invest. 1998 Feb 1;101(3):595-603. doi: 10.1172/JCI1333. J Clin Invest. 1998. PMID: 9449693 Free PMC article. - The role of gp130-mediated signals in osteoclast development: regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures.
Romas E, Udagawa N, Zhou H, Tamura T, Saito M, Taga T, Hilton DJ, Suda T, Ng KW, Martin TJ. Romas E, et al. J Exp Med. 1996 Jun 1;183(6):2581-91. doi: 10.1084/jem.183.6.2581. J Exp Med. 1996. PMID: 8676079 Free PMC article. - Modulation of osteoclast differentiation by local factors.
Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Suda T, et al. Bone. 1995 Aug;17(2 Suppl):87S-91S. doi: 10.1016/8756-3282(95)00185-g. Bone. 1995. PMID: 8579904 Review. - Mycoplasmal induction of cytokine production and major histocompatibility complex expression.
Stuart PM. Stuart PM. Clin Infect Dis. 1993 Aug;17 Suppl 1:S187-91. doi: 10.1093/clinids/17.supplement_1.s187. Clin Infect Dis. 1993. PMID: 8399913 Review.
Cited by
- Effect of a Single Injection of Benidipine-Impregnated Biodegradable Microcarriers on Bone and Gingival Healing at the Tooth Extraction Socket.
Imai M, Ayukawa Y, Yasunami N, Furuhashi A, Takemura Y, Adachi N, Hu J, Zhou X, Moriyama Y, Atsuta I, Kurata K, Koyano K. Imai M, et al. Adv Wound Care (New Rochelle). 2019 Mar 1;8(3):108-117. doi: 10.1089/wound.2018.0834. Epub 2019 Mar 5. Adv Wound Care (New Rochelle). 2019. PMID: 30911442 Free PMC article. - Interleukin-18 in Inflammatory Kidney Disease.
Hirooka Y, Nozaki Y. Hirooka Y, et al. Front Med (Lausanne). 2021 Mar 1;8:639103. doi: 10.3389/fmed.2021.639103. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33732720 Free PMC article. Review. - Close association between pulmonary disease manifestation in Mycoplasma pneumoniae infection and enhanced local production of interleukin-18 in the lung, independent of gamma interferon.
Narita M, Tanaka H, Abe S, Yamada S, Kubota M, Togashi T. Narita M, et al. Clin Diagn Lab Immunol. 2000 Nov;7(6):909-14. doi: 10.1128/CDLI.7.6.909-914.2000. Clin Diagn Lab Immunol. 2000. PMID: 11063497 Free PMC article. - The molecular mechanism of osteoclastogenesis in rheumatoid arthritis.
Udagawa N, Kotake S, Kamatani N, Takahashi N, Suda T. Udagawa N, et al. Arthritis Res. 2002;4(5):281-9. doi: 10.1186/ar431. Epub 2002 Apr 12. Arthritis Res. 2002. PMID: 12223101 Free PMC article. Review. - Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation.
Kamiya S, Nakamura C, Fukawa T, Ono K, Ohwaki T, Yoshimoto T, Wada S. Kamiya S, et al. J Bone Miner Metab. 2007;25(5):277-85. doi: 10.1007/s00774-007-0766-8. Epub 2007 Aug 25. J Bone Miner Metab. 2007. PMID: 17704992
References
- Suda, T., N. Takahashi, and T.J. Martin. 1995. Modulation of osteoclast differentiation: update. In Endocrine Review Monographs. Vol. 4. D.D. Bikle and A. Negrovilar, editors. Endocrine Society, Bethesda, MD. 266–270.
- Martin TJ, Ng KW. Mechanisms by which cells of the osteoblast lineage control osteoclast formation and activity. J Cell Biochem. 1994;56:357–366. - PubMed
- Suda, T., N. Udagawa, and N. Takahashi. 1996. Cells of bone: osteoclast generation. In Principles of Bone Biology. J.P. Billezikian, L.G. Raisz, G.A. Rodan, editors. Academic Press, San Diego, CA. 87–102.
- Roodman GD. Advances in bone biology: the osteoclast. Endocrine Rev. 1996;17:308–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous