A skin homing molecule defines the langerhans cell progenitor in human peripheral blood - PubMed (original) (raw)
A skin homing molecule defines the langerhans cell progenitor in human peripheral blood
D Strunk et al. J Exp Med. 1997.
Free PMC article
Abstract
We have recently described a system for the generation of dendritic cells (DC) and Langerhans cells (LC) from defined CD34+ precursors purified from peripheral blood of healthy adult volunteers. This study has now been extended by the characterization of two distinct subpopulations of CD34+ cells in normal human peripheral blood as defined by the expression of the skin homing receptor cutaneous lymphocyte-associated antigen (CLA). CD34+/CLA+ cells from normal peripheral blood were found to be CD71LOW/CD11a+/CD11b+/CD49d+/CD45RA+ whereas CD34+/CLA- cells displayed the CD71+/CD11aLOW/CD11bLOW/CD49d(+)/ CD45RA(LOW) phenotype. To determine the differentiation pathways of these two cell populations, CD34+ cells were sorted into CLA+ and CLA- fractions, stimulated with GM-CSF and TNF-alpha in vitro, and then were cultured for 10 to 18 d. Similar to unfractionated CD34+ cells, the progeny of both cell populations contained sizable numbers (12-22%) of dendritically shaped, CD1a+/HLA-DR cells. In addition to differences in their motility, the two dendritic cell populations generated differed from each other by the expression of LC-specific structures. Only the precursors expressing the skin homing receptor were found to differentiate into LC as evidenced by the presence of Birbeck granules. In contrast, CLA precursor cells generated a CD1a+ DC population devoid of Birbeck granule-containing LC. Provided that comparable mechanisms as found in this study are also operative in vivo, we postulate that the topographic organization of the DC system is already determined, at least in part, at the progenitor level.
Figures
Figure 1
Distinct phenotype of CLA+ and CLA− PBPC in normal peripheral blood. Using three-color flow cytometry, freshly isolated PBPC from healthy volunteers were analyzed for the distribution of the antigens shown on the y-axis. Reactivity was measured for gated CLA+/ CD34+ (black bars) and CLA-/CD34+ (hatched bars) PBPC. Results of one experiment are displayed as mean fluorescence intensity (MFI). Two additional experiments with cells from different donors yielded similar results. The lack of anti-CD1a-reactivity was used as a negative control.
Figure 2
Immunohistochemical analysis of CLA+ and CLA− PBPC progeny. CLA+ and CLA− PBPC were cultured for two weeks in the presence of GM-CSF/TNF-α and then subjected to Lag immunolabeling using the APAAP procedure. Clustered (asterisks) as well as single (arrows) Lag+ cells (red) were detectable within the CLA+/CD34+ progeny (A). No Lag reactivity was found within the progeny of CLA− PBPC (B). Original magnification, ×150.
Figure 3
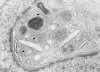
Detection of Birbeck granules in CD1a+ cells derived from CLA+ progenitors. Representative ultramorphology of a dendritically shaped CD1a+ LC that was generated from CLA+ PBPC during 16 d of culture in the presence of GM-CSF and TNF-α. Numerous trilaminar Birbeck granules (arrowheads) can be easily seen in the perinuclear area. Original magnification, ×83,500.
Figure 4
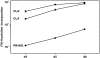
MLR-stimulatory capacity of cells derived from CLA+ and CLA− PBPC. 3.3 × 103 cells generated in a 2-wk culture in GM-CSF/ TNF–containing medium from CLA+ (circles) and CLA− (diamonds) PBPC and, for comparison, monocytes from the same donor (squares) were tested for their capacity to stimulate 105 allogeneic T cells. Cells were pulsed with [3H]thymidine for 16 h after 4, 5, or 6 d of coculture. The [3H]thymidine incorporation rate is expressed as mean cpm values ± SD of triplicate cultures. 105 mitomycin C–treated APC or purified T cells gave <500 cpm. One representative experiment out of three performed is shown.
Figure 5
A subpopulation of cells derived from CLA+/CD34+ PBPC exhibits a high motility in culture. Time-lapse microphotography was performed with cells derived from CLA+ PBPC after 3 wk of culture in the presence of GM-CSF/TNF-α. Pictures of cells migrating within the culture well were taken every minute. One (arrowhead) of the two dendritically shaped cells shown (A, time point zero) covers a distance being multiples of its own diameter within 3 min (B). Original magnification, ×150.
Figure 5
A subpopulation of cells derived from CLA+/CD34+ PBPC exhibits a high motility in culture. Time-lapse microphotography was performed with cells derived from CLA+ PBPC after 3 wk of culture in the presence of GM-CSF/TNF-α. Pictures of cells migrating within the culture well were taken every minute. One (arrowhead) of the two dendritically shaped cells shown (A, time point zero) covers a distance being multiples of its own diameter within 3 min (B). Original magnification, ×150.
Similar articles
- CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner.
Jaksits S, Kriehuber E, Charbonnier AS, Rappersberger K, Stingl G, Maurer D. Jaksits S, et al. J Immunol. 1999 Nov 1;163(9):4869-77. J Immunol. 1999. PMID: 10528188 - In vitro differentiation of CD34+ hematopoietic progenitor cells toward distinct dendritic cell subsets of the birbeck granule and MIIC-positive Langerhans cell and the interdigitating dendritic cell type.
Herbst B, Köhler G, Mackensen A, Veelken H, Kulmburg P, Rosenthal FM, Schaefer HE, Mertelsmann R, Fisch P, Lindemann A. Herbst B, et al. Blood. 1996 Oct 1;88(7):2541-8. Blood. 1996. PMID: 8839846 - Delineation of the dendritic cell lineage by generating large numbers of Birbeck granule-positive Langerhans cells from human peripheral blood progenitor cells in vitro.
Mackensen A, Herbst B, Köhler G, Wolff-Vorbeck G, Rosenthal FM, Veelken H, Kulmburg P, Schaefer HE, Mertelsmann R, Lindemann A. Mackensen A, et al. Blood. 1995 Oct 1;86(7):2699-707. Blood. 1995. PMID: 7545468 - [Cutaneous immune system].
Schmitt D. Schmitt D. C R Seances Soc Biol Fil. 1994;188(3):207-21. C R Seances Soc Biol Fil. 1994. PMID: 7834504 Review. French. - [The Langerhans cell: from in vitro production to use in cellular immunotherapy].
Schmitt D. Schmitt D. J Soc Biol. 2001;195(1):69-74. J Soc Biol. 2001. PMID: 11530504 Review. French.
Cited by
- Batch Effects during Human Bone Marrow Stromal Cell Propagation Prevail Donor Variation and Culture Duration: Impact on Genotype, Phenotype and Function.
Brachtl G, Poupardin R, Hochmann S, Raninger A, Jürchott K, Streitz M, Schlickeiser S, Oeller M, Wolf M, Schallmoser K, Volk HD, Geissler S, Strunk D. Brachtl G, et al. Cells. 2022 Mar 10;11(6):946. doi: 10.3390/cells11060946. Cells. 2022. PMID: 35326396 Free PMC article. - Identification of CD318, TSPAN8 and CD66c as target candidates for CAR T cell based immunotherapy of pancreatic adenocarcinoma.
Schäfer D, Tomiuk S, Küster LN, Rawashdeh WA, Henze J, Tischler-Höhle G, Agorku DJ, Brauner J, Linnartz C, Lock D, Kaiser A, Herbel C, Eckardt D, Lamorte M, Lenhard D, Schüler J, Ströbel P, Missbach-Guentner J, Pinkert-Leetsch D, Alves F, Bosio A, Hardt O. Schäfer D, et al. Nat Commun. 2021 Mar 5;12(1):1453. doi: 10.1038/s41467-021-21774-4. Nat Commun. 2021. PMID: 33674603 Free PMC article. - 3D skin models in domestic animals.
Souci L, Denesvre C. Souci L, et al. Vet Res. 2021 Feb 15;52(1):21. doi: 10.1186/s13567-020-00888-5. Vet Res. 2021. PMID: 33588939 Free PMC article. Review. - Dendritic cells in the pathogenesis of sarcoidosis.
Zaba LC, Smith GP, Sanchez M, Prystowsky SD. Zaba LC, et al. Am J Respir Cell Mol Biol. 2010 Jan;42(1):32-9. doi: 10.1165/rcmb.2009-0033TR. Epub 2009 Apr 16. Am J Respir Cell Mol Biol. 2010. PMID: 19372243 Free PMC article. Review. - HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells.
Schuster C, Vaculik C, Fiala C, Meindl S, Brandt O, Imhof M, Stingl G, Eppel W, Elbe-Bürger A. Schuster C, et al. J Exp Med. 2009 Jan 16;206(1):169-81. doi: 10.1084/jem.20081747. Epub 2009 Jan 12. J Exp Med. 2009. PMID: 19139172 Free PMC article.
References
- Strunk D, Rappersberger K, Egger C, Strobl H, Krömer E, Elbe A, Maurer D, Stingl G. Generation of human dendritic cells/Langerhans cells from circulating CD34+hematopoietic progenitor cells. Blood. 1996;87:1292–1302. - PubMed
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
- Aiba S, Katz SI. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J Immunol. 1990;145:2791–2796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials