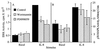Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases - PubMed (original) (raw)
Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases
C Knall et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Chemoattractants and chemokines, such as interleukin 8 (IL-8), are defined by their ability to induce directed cell migration of responsive cells. The signal transduction pathway(s) leading to cell migration remain ill defined. We demonstrate that phosphatidylinositol-3-kinase (PI3K) activity, as determined by inhibition using wortmannin and LY294002, is required for IL-8-induced cell migration of human neutrophils. Recently we reported that IL-8 caused the activation of the Ras/Raf/extracellular signal-regulated kinase (ERK) pathway in human neutrophils and that this activation was dependent on PI3K activity. The regulation of cell migration by IL-8 is independent of ERK kinase and ERK activation since the ERK kinase inhibitor PD098059 had no effect on IL-8-induced cell migration of human neutrophils. Additionally, activation of p38-mitogen-activated protein kinase is insufficient and activation of c-Jun N-terminal kinase is unnecessary to induce cell migration of human neutrophils. Therefore, regulation of neutrophil migration appears to be largely independent of the activation of the mitogen-activated protein kinases. The data argue that PI3K activity plays a central role in multiple signal transduction pathways within the human neutrophil leading to distinct cell functions.
Figures
Figure 1
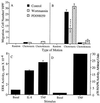
IL-8 stimulated activation of ERK but not p38-MAPK is sensitive to wortmannin and PD098059. Human neutrophils were treated with either the carrier dimethyl sulfoxide alone, 100 nM wortmannin for 10 min or 50 μM PD098059 for 1 hr prior to stimulation with 25 nM IL-8. (A) Samples were stimulated for 3 min then assayed for ERK activity. The data shown represent the mean ± SEM of triplicate measurements of ERK activity for each sample and are representative of more than six independent experiments. ∗, P = 0.0126; ∗∗, P = 0.0066; ∗∗∗, P = 0.0003. (B) Samples were stimulated for 10 min then assayed for p38-MAPK activity. The data shown represent the mean ± SEM of PhosphorImager data from five independent experiments.
Figure 2
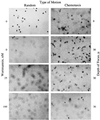
Wortmannin inhibits IL-8-induced cell migration of human neutrophils. Freshly isolated neutrophils were treated with the indicated concentrations of wortmannin or carrier alone for 10 min prior to their use in the chemotaxis assay. Random motion was measured in the presence of KRPD-HSA only. Chemotactic motion was measured in the presence of KRPD-HSA containing 2.5 nM IL-8 (a maximal dose for neutrophil chemotaxis, data not shown) in the lower chamber of the blind well Boyden chamber. Migration was measured at a depth of 30 μm. The photomicrographs shown are of individual filters representative of triplicate filters analyzed for each sample. (×400.)
Figure 3
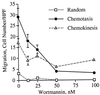
Wortmannin inhibits IL-8-induced cell migration with an IC50 of 26 nM. This figure shows the numerical analysis of the chemotaxis assay presented in Fig. 2. Chemokinetic motion was measured in the presence of KRPD-HSA containing 2.5 nM IL-8 in both the lower and upper chambers of the blind well Boyden chamber. The data shown represent the mean ± SEM of cell counts taken from nine high power fields (three fields from each replicate filter) at a depth of 30 μm from the top of the filter.
Figure 4
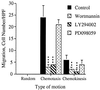
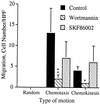
Both wortmannin and LY294002 but not PD098059 inhibit IL-8-induced cell migration. Human neutrophils were treated with either carrier alone, 100 nM wortmannin for 10 min, 50 μM LY294002 for 5 min or 50 μM PD098059 for 1 hr prior to their use in the chemotaxis assay. The data shown represent the mean ± SEM of cell counts taken from nine high power fields (3 fields from each replicate filter) at a depth of 60 μm from the top of the filter and are representative of at least three independent experiments. ∗∗∗, P < 0.0001; ∗∗, P = 0.0064. The assays were performed as described in the legends to Figs. 2 and 3 and in Materials and Methods.
Figure 5
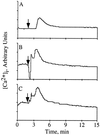
Wortmannin and PD098059 do not inhibit IL-8 stimulated calcium mobilization. Human neutrophils were loaded with 5 μM Indo-1 AM for 1 hr and simultaneously incubated with either carrier alone (A), 100 nM wortmannin for the final 10 min (B) or 50 μM PD098059 for 1 hr (C). [Ca2+]i was determined by flow microfluorimetry using a cytofluorograf 50H (Ortho Diagnostics) with
acqcyte
software (Phoenix Flow Systems, San Diego). Samples were analyzed for 2 min prior to stimulation to establish the basal [Ca2+]i. IL-8, 25 nM in KRPD-HSA was then added to the sample, indicated by the arrow, and data collection was continued for an additional 13 min. The data was analyzed using
mtime
software (Phoenix Flow Systems). The data shown are representative of five independent experiments. The increased resting levels of calcium in B and C are due to incomplete removal from the flow cytometer of IL-8 used to stimulate the previous sample as determined by analyzing the samples in different orders (data not shown).
Figure 6
TNF does not stimulate cell migration of human neutrophils but does stimulate ERK and p38-MAPK activation. (A and B) Human neutrophils were treated with either carrier alone, 100 nM wortmannin for 10 min or 50 μM PD098059 for 1 hr prior to their use in the chemotaxis assay. Random motion was measured in the presence of KRPD-HSA only. Chemotactic motion was measured in the presence of KRPD-HSA containing either 10 ng/ml TNF (A) or 2.5 nM IL-8 (B) in the lower chamber of the blind well Boyden chamber. Chemokinetic motion was measured in the presence of KRPD-HSA containing either 10 ng/ml TNF (A) or 2.5 nM IL-8 (B) in both the lower and upper chambers of the blind well Boyden chamber. The data shown represent the mean ± SEM of cell counts taken from nine high power fields (three fields from each replicate filter) at a depth of 60 μm from the top of the filter. ∗∗∗, P = 0.0005. (C and D) Samples were stimulated with 10 ng/ml TNF for 10 min and then assayed for ERK activity (C) or p38-MAPK activity (D). (C) The data shown represent the mean ± SEM of triplicate measurements of ERK activity for each sample. (D) The PhosphorImager data shown are representative of at least three independent experiments.
Figure 7
SK&F 86002 does not inhibit IL-8-induced cell migration. Human neutrophils were treated with either carrier alone, 100 nM wortmannin for 10 min, or 10 μM SK&F 86002 for 1 hr prior to their use in the chemotaxis assay. The data shown represent the mean ± SEM of the mean cell counts from three independent experiments performed and analyzed as described in the legend to Figs. 2, 3, 4 and Materials and Methods. ∗∗, P = 0.0041; ∗, P = 0.0198.
Similar articles
- Comparison of the roles of mitogen-activated protein kinase kinase and phosphatidylinositol 3-kinase signal transduction in neutrophil effector function.
Coffer PJ, Geijsen N, M'rabet L, Schweizer RC, Maikoe T, Raaijmakers JA, Lammers JW, Koenderman L. Coffer PJ, et al. Biochem J. 1998 Jan 1;329 ( Pt 1)(Pt 1):121-30. doi: 10.1042/bj3290121. Biochem J. 1998. PMID: 9405284 Free PMC article. - Chemotactic peptide-induced activation of MEK-2, the predominant isoform in human neutrophils. Inhibition by wortmannin.
Downey GP, Butler JR, Brumell J, Borregaard N, Kjeldsen L, Sue-A-Quan AK, Grinstein S. Downey GP, et al. J Biol Chem. 1996 Aug 30;271(35):21005-1011. doi: 10.1074/jbc.271.35.21005. J Biol Chem. 1996. PMID: 8702863 - Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation.
King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. King WG, et al. Mol Cell Biol. 1997 Aug;17(8):4406-18. doi: 10.1128/MCB.17.8.4406. Mol Cell Biol. 1997. PMID: 9234699 Free PMC article. - Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2.
Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Hodge C, et al. J Biol Chem. 1998 Nov 20;273(47):31327-36. doi: 10.1074/jbc.273.47.31327. J Biol Chem. 1998. PMID: 9813041
Cited by
- CXCR1/2 antagonism with CXCL8/Interleukin-8 analogue CXCL8(3-72)K11R/G31P restricts lung cancer growth by inhibiting tumor cell proliferation and suppressing angiogenesis.
Khan MN, Wang B, Wei J, Zhang Y, Li Q, Luan X, Cheng JW, Gordon JR, Li F, Liu H. Khan MN, et al. Oncotarget. 2015 Aug 28;6(25):21315-27. doi: 10.18632/oncotarget.4066. Oncotarget. 2015. PMID: 26087179 Free PMC article. - PAK1 kinase is required for CXCL1-induced chemotaxis.
Wang D, Sai J, Carter G, Sachpatzidis A, Lolis E, Richmond A. Wang D, et al. Biochemistry. 2002 Jun 4;41(22):7100-7. doi: 10.1021/bi025902m. Biochemistry. 2002. PMID: 12033944 Free PMC article. - Enhanced neutrophil motility by granulocyte colony-stimulating factor: the role of extracellular signal-regulated kinase and phosphatidylinositol 3-kinase.
Nakamae-Akahori M, Kato T, Masuda S, Sakamoto E, Kutsuna H, Hato F, Nishizawa Y, Hino M, Kitagawa S. Nakamae-Akahori M, et al. Immunology. 2006 Nov;119(3):393-403. doi: 10.1111/j.1365-2567.2006.02448.x. Epub 2006 Aug 14. Immunology. 2006. PMID: 16903868 Free PMC article. - Evaluation of signal transduction pathways in chemoattractant-induced human monocyte chemotaxis.
Fine JS, Byrnes HD, Zavodny PJ, Hipkin RW. Fine JS, et al. Inflammation. 2001 Apr;25(2):61-7. doi: 10.1023/a:1007152903135. Inflammation. 2001. PMID: 11321360 - Neutrophil cell surface receptors and their intracellular signal transduction pathways.
Futosi K, Fodor S, Mócsai A. Futosi K, et al. Int Immunopharmacol. 2013 Nov;17(3):638-50. doi: 10.1016/j.intimp.2013.06.034. Epub 2013 Aug 30. Int Immunopharmacol. 2013. PMID: 23994464 Free PMC article. Review.
References
- Lauffenburger D A, Horwitz A F. Cell. 1996;84:359–369. - PubMed
- Mitchison T J, Cramer L P. Cell. 1996;84:371–379. - PubMed
- Ridley A J, Hall A. Cell. 1992;70:389–399. - PubMed
- Ridley A J, Paterson H F, Johnston C L, Diekman D, Hall A. Cell. 1992;70:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM30324/GM/NIGMS NIH HHS/United States
- R01 DK037871/DK/NIDDK NIH HHS/United States
- R01 GM030324/GM/NIGMS NIH HHS/United States
- DK37871/DK/NIDDK NIH HHS/United States
- DK48845/DK/NIDDK NIH HHS/United States
- F32 HL009640/HL/NHLBI NIH HHS/United States
- R37 GM030324/GM/NIGMS NIH HHS/United States
- P01 HL034303/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous