Target of rapamycin proteins and their kinase activities are required for meiosis - PubMed (original) (raw)
Target of rapamycin proteins and their kinase activities are required for meiosis
X F Zheng et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The phosphatidylinositol kinase-related kinases, including Tor1p, Tor2p, FRAP/RAFT, FRP/ATR, ATM, Mec1p, Rad3, and Tel1p, function in signal transduction pathways involved in cell cycle progression and surveillance. The rapamycin-sensitive kinase activities of Tor1p and Tor2p are required for the nutrient-activated protein translation essential for G1 cell cycle progression in haploid yeast cells. In addition, Tor2p's kinase activity is necessary for its unique rapamycin-insensitive function involved in the assembly of the actin cytoskeleton. In the current study using diploid yeast, we found that the kinase activities of the Tor proteins are also required for two discrete steps during yeast meiosisthe switch between the mitotic and meiotic cell cycles and a later step during meiosis involved in the packaging of resultant haploid cells (spores) into asci. Based on what is known of the mitotic functions of Tor and FRAP proteins, these results likely reflect the requirement for signaling pathways leading to regulated protein translation during meiosis. Mec1p, which is required for meiotic recombination, and the Tor proteins are, therefore, homologous kinases with distinct, yet essential, roles in meiosis.
Figures
Figure 1
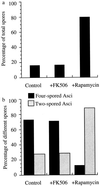
(a) Rapamycin causes an increase in sporulation efficiency in BR2495 cells. Diploid yeast BR2495 cells were grown to saturation in YPD medium for 24 hr at 30°C with vigorous shaking. These cells were added to YPA medium at a 1:50 dilution and incubated for a further 13.5 hr at 30°C. The cells were then pelleted, washed twice with sterile water, resuspended into SPM with or without rapamycin or FK506, and incubated for another 36 hr. Different asci were counted under the phase-contrast light microscope. The results from three different experiments were averaged (n = 250). Control, methanol (solvent for both FK506 and rapamycin); FK506, 100 nM final concentration; rapamycin, 100 nM final concentration. (b) Increased dyad population in the presence of rapmycin. The same samples were counted for proportions of different ascospores. The percentage of single-spored asci (monads) was very low (<1%) and omitted from the results. A small percentage of three-spored asci (triads) were counted as four-spored asci (tetrads) in this analysis. The results show an average of three different experiments (n = 250).
Figure 2
(a) Rapamycin induces SK-1 cells to sporulate in saturated YPD culture. Diploid SK-1 cells were incubated in YPD for 24 hr at 30°C with vigorous shaking. The cells reached saturation and arrested in G1. The cell culture was equally divided into three aliquots. Each aliquot was incubated with methanol (control), 100 nM FK506 (YPD + FK506), or 100 nM rapamycin (YPD + rapamycin). As a positive control, the same saturated SK-1 cell culture in YPD was diluted 1:50 into YPA and incubated for 13.5 hr. The cells were washed with sterile water, resuspended into SPM, and incubated for another 13 hr. The proportion of total sporulated cells was calculated as in Fig. 1_a_ (average of three experiments, n = 250). (b) Overexpression of plasmid-borne wild-type, but not the kinase-inactive mutant TOR1, partially blocks sporulation. The diploid SK-1 cells carrying pGAL1 alone, pGAL1-TOR1, or pGAL1-TOR1 (Asp-2294 → Glu) were incubated in complete synthetic medium-Leu-Glu for 36 hr at 30°C with vigorous shaking, washed twice in sterile water, and resuspended into the 50 vol of YPA for another 10 hr. Galactose (0.1%) was added to the culture. The cells were incubated for another 3.5 hr during protein induction, then washed twice with sterile water, resuspended in SPM containing 0.1% galactose, and incubated for 13 hr. The samples were analyzed as in a (average of three experiments, n = 250).
Figure 3
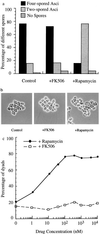
(a) Rapamycin causes unusually high proportion of two-spored asci. Diploid SK-1 cells were incubated in YPD for 24 hr at 30°C with vigorous shaking and then diluted 1:50 into YPA. After 13.5 hr of further incubation, the cells were washed twice with sterile water and resuspended into SPM supplemented with methanol (control), 100 nM FK506 (+ FK506), or 100 nM rapamycin (+ rapamycin) for another 13 hr. Proportions of the different asci were counted (n = 250). The results shown were averaged from three different experiments. (b) The control and FK506- and rapamycin-treated spores under the light microscope (×1,000). (c) Dosage effects of FK506 and rapamycin on sporulation. Diploid SK-1 cells were cultured in YPD and grown to saturation density for 24 hr at 30°C with vigorous shaking. These cells were then diluted 1:50 into YPA. After 13.5 hr of further incubation, the cells were washed twice with sterile water and resuspended into SPM supplemented with 0, 1, 3, 10, 30, 100, 300, 1,000, 3,000, and 10,000 nM FK506 or rapamycin, respectively. After incubation for another 13 hr, the proportion of two-spored asci was counted (n = 250). The results shown were averaged from three different experiments.
Figure 4
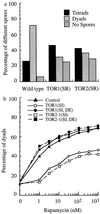
(a) TOR1 (Ser-1972 → Arg) or TOR2 (Ser-1975 → Arg) renders dominant rapamycin resistance during meiosis. Wild-type diploid SK-1 [a/α, _TOR1/TOR1, TOR2/TOR2_] or SK-1 with chromosomal rapamycin-resistant mutations Ser-1972 → Arg in TOR1 [a/α, TOR1/TOR1 (Ser-1972 → Arg), TOR2/TOR2_] or Ser-1975 → Arg in TOR2 [a/α, TOR1/TOR1, TOR2/TOR2 (Ser-1975 → Arg)] cells were incubated in YPD for 24 hr at 30°C with vigorous shaking. These cells were then diluted 1:50 into YPA. After 13.5 hr of further incubation, the cells were washed twice with sterile water and resuspended into SPM supplemented with 100 nM rapamycin. The samples were analyzed as in Fig. 3_a. (b) The kinase activities of Tor proteins are required for rapamycin-sensitive meiotic functions. Diploid SK-1 strains containing a control plasmid, a plasmid expressing TOR1 (Ser-1972 → Ile), TOR1 (Ser-1972 → Ile, Asp-2294 → Glu), TOR2–1 (Ser-1972 → Ile), and TOR2–1 (Ser-1972 → Ile, Asp-2294 → Glu), respectively, were incubated in selection medium for 36 hr to saturation, diluted 1:50 into YPA for another 13 hr before being resuspended into SPM supplemented with different concentrations of rapamycin for a further 13.5 hr. The results represent an average of three different experiments (n = 250).
Figure 5
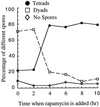
Timing of the late rapamycin-sensitive step during late meiosis. Diploid SK-1 cells were incubated in YPD for 24 hr at 30°C with vigorous shaking. These cells were then diluted 1:50 into YPA. After 13.5 hr of further incubation, they were washed twice with sterile water and resuspended into SPM (0 hr), and then 100 nM rapamycin was added to various culture aliquots at 0, 2, 4, 6, 8, and 10 hr, and the proportion of different asci in each sample was analyzed as in Fig. 3_a_.
Figure 6
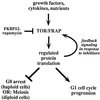
Outline of TOR/FRAP pathways. Extracellular signals pass through the Tor and FRAP proteins, suppressing quiescence or meiosis and promoting cell cycle progression. The pathway can also be activated with cell-permeable RNA ligands that inhibit protein synthesis, a process reminiscent of the activation of the Mec1p-mediated pathway by cell-permeable DNA ligands or by inhibitors of DNA synthesis (2).
Similar articles
- Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins.
Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL. Hardwick JS, et al. Proc Natl Acad Sci U S A. 1999 Dec 21;96(26):14866-70. doi: 10.1073/pnas.96.26.14866. Proc Natl Acad Sci U S A. 1999. PMID: 10611304 Free PMC article. - Protein kinase activity and identification of a toxic effector domain of the target of rapamycin TOR proteins in yeast.
Alarcon CM, Heitman J, Cardenas ME. Alarcon CM, et al. Mol Biol Cell. 1999 Aug;10(8):2531-46. doi: 10.1091/mbc.10.8.2531. Mol Biol Cell. 1999. PMID: 10436010 Free PMC article. - TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin.
Zheng XF, Florentino D, Chen J, Crabtree GR, Schreiber SL. Zheng XF, et al. Cell. 1995 Jul 14;82(1):121-30. doi: 10.1016/0092-8674(95)90058-6. Cell. 1995. PMID: 7606777 - Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae.
Crespo JL, Hall MN. Crespo JL, et al. Microbiol Mol Biol Rev. 2002 Dec;66(4):579-91, table of contents. doi: 10.1128/MMBR.66.4.579-591.2002. Microbiol Mol Biol Rev. 2002. PMID: 12456783 Free PMC article. Review. - Identification of TOR signaling complexes: more TORC for the cell growth engine.
Abraham RT. Abraham RT. Cell. 2002 Oct 4;111(1):9-12. doi: 10.1016/s0092-8674(02)01009-7. Cell. 2002. PMID: 12372295 Review.
Cited by
- Disruption of the Schizosaccharomyces japonicus lig4 Disturbs Several Cellular Processes and Leads to a Pleiotropic Phenotype.
Acs-Szabo L, Papp LA, Takacs S, Miklos I. Acs-Szabo L, et al. J Fungi (Basel). 2023 May 10;9(5):550. doi: 10.3390/jof9050550. J Fungi (Basel). 2023. PMID: 37233261 Free PMC article. - The Beginning of Meiosis in Mammalian Female Germ Cells: A Never-Ending Story of Intrinsic and Extrinsic Factors.
Farini D, De Felici M. Farini D, et al. Int J Mol Sci. 2022 Oct 20;23(20):12571. doi: 10.3390/ijms232012571. Int J Mol Sci. 2022. PMID: 36293427 Free PMC article. Review. - Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins.
Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL. Hardwick JS, et al. Proc Natl Acad Sci U S A. 1999 Dec 21;96(26):14866-70. doi: 10.1073/pnas.96.26.14866. Proc Natl Acad Sci U S A. 1999. PMID: 10611304 Free PMC article. - SPO21 is required for meiosis-specific modification of the spindle pole body in yeast.
Bajgier BK, Malzone M, Nickas M, Neiman AM. Bajgier BK, et al. Mol Biol Cell. 2001 Jun;12(6):1611-21. doi: 10.1091/mbc.12.6.1611. Mol Biol Cell. 2001. PMID: 11408572 Free PMC article. - Control of meiosis by respiration.
Jambhekar A, Amon A. Jambhekar A, et al. Curr Biol. 2008 Jul 8;18(13):969-75. doi: 10.1016/j.cub.2008.05.047. Curr Biol. 2008. PMID: 18595705 Free PMC article.
References
- Keith C T, Schreiber S L. Science. 1995;270:50–51. - PubMed
- Brown E J, Schreiber S L. Cell. 1996;86:517–520. - PubMed
- Chung J, Kuo C, Crabtree G R, Blenis J. Cell. 1992;69:1227–1236. - PubMed
- Keegan K, Holtzman D, Plug A, Christenson E, Brainerd E, Flaggs G, Bentley N, Taylor E, Meyn M, Moss S, Carr A, Ashley T, Hoekstra M. Genes Dev. 1996;10:2423–2437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous