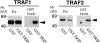TRAF-interacting protein (TRIP): a novel component of the tumor necrosis factor receptor (TNFR)- and CD30-TRAF signaling complexes that inhibits TRAF2-mediated NF-kappaB activation - PubMed (original) (raw)
Comparative Study
TRAF-interacting protein (TRIP): a novel component of the tumor necrosis factor receptor (TNFR)- and CD30-TRAF signaling complexes that inhibits TRAF2-mediated NF-kappaB activation
S Y Lee et al. J Exp Med. 1997.
Abstract
Through their interaction with the TNF receptor-associated factor (TRAF) family, members of the tumor necrosis factor receptor (TNFR) superfamily elicit a wide range of biological effects including differentiation, proliferation, activation, or cell death. We have identified and characterized a novel component of the receptor-TRAF signaling complex, designated TRIP (TRAF-interacting protein), which contains a RING finger motif and an extended coiled-coil domain. TRIP associates with the TNFR2 or CD30 signaling complex through its interaction with TRAF proteins. When associated, TRIP inhibits the TRAF2-mediated NF-kappaB activation that is required for cell activation and also for protection against apoptosis. Thus, TRIP acts as a receptor-proximal regulator that may influence signals responsible for cell activation/proliferation and cell death induced by members of the TNFR superfamily.
Figures
Figure 1
Interaction of TRIP with TRAF1 and TRAF2. An expression vector encoding TRAF1 or TRAF2 was co-transfected into 293 cells with an expression vector encoding either GST alone or GST–TRIP fusion proteins. After 36 h, cell lysates were prepared and subjected to purification with glutathione–Sepharose beads. Proteins coprecipitated with GST fusion proteins were analyzed by Western blot analysis with antiTRAF1 or anti-TRAF2 polyclonal antibodies. (Pre) Cell lysates before precipitation with glutathione beads were analyzed by Western blot analysis to show that similar amounts of TRAF1 or TRAF2 are present in each sample. (GST beads) Proteins coprecipitated with GST-fusion proteins were analyzed. TRAF1 or TRAF2 are indicated with arrows.
Figure 2
Predicted amino acid sequences of mTRIP and hTRIP. (A) The full length mouse sequence is shown and numbered. The human sequence has one less amino acid than that of the mouse (indicated with a dot at position 302). Dashes indicate positions in the human sequence which are identical to those in the mouse. Cysteine and histidine residues defining the RING finger motif are marked by boxes. Brackets indicate the potential coiled-coil region of TRIP. Within the brackets, amino acids that form the putative coiled-coil structures are marked by overlying dots, and those that form leucine-zipper structures are indicated in bold. The accession numbers for the mouse and human TRIP sequences reported in this paper are U77844 and U77845, respectively. (B) Comparison of amino acid sequences from various proteins that contain RING finger motifs. The RING finger domains of mTRIP and hTRIP are aligned with those of TRAFs, c-IAP1, human protooncogene c-cbl, human RING1, human ribonucleoprotein SS-A/Ro, chicken C-RZF, and Drosophila neuralized gene (38). Residues corresponding to the consensus sequence are indicated in bold. (C) Helical wheel representation of residues 225 to 260 of TRIP. The wheel starts with the inner residue Leu225 at position d and finishes with the outer residue Ala260 at position d.
Figure 2
Predicted amino acid sequences of mTRIP and hTRIP. (A) The full length mouse sequence is shown and numbered. The human sequence has one less amino acid than that of the mouse (indicated with a dot at position 302). Dashes indicate positions in the human sequence which are identical to those in the mouse. Cysteine and histidine residues defining the RING finger motif are marked by boxes. Brackets indicate the potential coiled-coil region of TRIP. Within the brackets, amino acids that form the putative coiled-coil structures are marked by overlying dots, and those that form leucine-zipper structures are indicated in bold. The accession numbers for the mouse and human TRIP sequences reported in this paper are U77844 and U77845, respectively. (B) Comparison of amino acid sequences from various proteins that contain RING finger motifs. The RING finger domains of mTRIP and hTRIP are aligned with those of TRAFs, c-IAP1, human protooncogene c-cbl, human RING1, human ribonucleoprotein SS-A/Ro, chicken C-RZF, and Drosophila neuralized gene (38). Residues corresponding to the consensus sequence are indicated in bold. (C) Helical wheel representation of residues 225 to 260 of TRIP. The wheel starts with the inner residue Leu225 at position d and finishes with the outer residue Ala260 at position d.
Figure 2
Predicted amino acid sequences of mTRIP and hTRIP. (A) The full length mouse sequence is shown and numbered. The human sequence has one less amino acid than that of the mouse (indicated with a dot at position 302). Dashes indicate positions in the human sequence which are identical to those in the mouse. Cysteine and histidine residues defining the RING finger motif are marked by boxes. Brackets indicate the potential coiled-coil region of TRIP. Within the brackets, amino acids that form the putative coiled-coil structures are marked by overlying dots, and those that form leucine-zipper structures are indicated in bold. The accession numbers for the mouse and human TRIP sequences reported in this paper are U77844 and U77845, respectively. (B) Comparison of amino acid sequences from various proteins that contain RING finger motifs. The RING finger domains of mTRIP and hTRIP are aligned with those of TRAFs, c-IAP1, human protooncogene c-cbl, human RING1, human ribonucleoprotein SS-A/Ro, chicken C-RZF, and Drosophila neuralized gene (38). Residues corresponding to the consensus sequence are indicated in bold. (C) Helical wheel representation of residues 225 to 260 of TRIP. The wheel starts with the inner residue Leu225 at position d and finishes with the outer residue Ala260 at position d.
Figure 3
TRIP mRNA expression in mouse tissues and its regulation during cell activation. (A) Northern blot analysis of TRIP mRNA in mouse tissues. Total RNA isolated from various tissues was hybridized with a _TRIP_-specific probe. The TRIP probe hybridized to an ∼2.1-kb mRNA, indicated by the arrow. Positions of 18S and 28S ribosomal RNA are indicated. The amount of total RNA loaded in each lane was similar based on the intensity of EtBr-stained rRNAs (data not shown). (B) Expression of TRAF, c-IAP1, and TRIP during lymphocyte stimulation. cDNAs were prepared from lymph node cells stimulated with antiTCR Ab plus anti-CD28 Abs for 0 (cont.) and 48 (activ.) h. The cDNAs were then subjected to semiquantitative PCR using primers specific for mTRIP, mTRAF1, and m-c-IAP1 as described in Materials and Methods.
Figure 3
TRIP mRNA expression in mouse tissues and its regulation during cell activation. (A) Northern blot analysis of TRIP mRNA in mouse tissues. Total RNA isolated from various tissues was hybridized with a _TRIP_-specific probe. The TRIP probe hybridized to an ∼2.1-kb mRNA, indicated by the arrow. Positions of 18S and 28S ribosomal RNA are indicated. The amount of total RNA loaded in each lane was similar based on the intensity of EtBr-stained rRNAs (data not shown). (B) Expression of TRAF, c-IAP1, and TRIP during lymphocyte stimulation. cDNAs were prepared from lymph node cells stimulated with antiTCR Ab plus anti-CD28 Abs for 0 (cont.) and 48 (activ.) h. The cDNAs were then subjected to semiquantitative PCR using primers specific for mTRIP, mTRAF1, and m-c-IAP1 as described in Materials and Methods.
Figure 4
Mapping of TRIP–TRAF interaction domains. (A) Interaction of TRAF1 or TRAF2 with the NH2- and COOH-terminal domains of TRIP. Expression vectors encoding wild-type TRIP or the indicated deletion mutants of TRIP fused to the transcription activation domain were cotransformed into yeast with plasmids expressing LexA DNA binding domain–TRAF1 or –TRAF2 fusion proteins. Interactions between fusion proteins were scored by measuring β-gal activity of yeast transformants. (+) Average β-gal activity of three independent yeast transformants was higher than 1,000 Miller units; (−) average β-gal activity of three independent yeast transformants was about 100 Miller units, which was similar to that of negative controls (bait plasmid alone). (B) Interaction of TRIP with TRAFs. Expression vectors encoding the NH2-terminal deletion mutants of TRAF fused to the LexA DNA binding domain were co-transformed into yeast with plasmids expressing TRIP fused to the transcription activation domain. Interactions between fusion proteins were scored by measuring β-gal activity of yeast transformants as described in Fig. 4_A._ For the analysis of the COOH-terminal deletion mutants of TRAFs, a transient transfection-based coprecipitation experiment was used. The indicated COOH-terminal deletion mutants of TRAFs were coexpressed with GST–TRIP fusion proteins in 293 cells. Cell lysates were subjected for purification with glutathione–Sepharose beads, followed by Western blot analysis with anti-TRAF1 or anti-TRAF2 polyclonal antibodies as described in Fig. 1.
Figure 4
Mapping of TRIP–TRAF interaction domains. (A) Interaction of TRAF1 or TRAF2 with the NH2- and COOH-terminal domains of TRIP. Expression vectors encoding wild-type TRIP or the indicated deletion mutants of TRIP fused to the transcription activation domain were cotransformed into yeast with plasmids expressing LexA DNA binding domain–TRAF1 or –TRAF2 fusion proteins. Interactions between fusion proteins were scored by measuring β-gal activity of yeast transformants. (+) Average β-gal activity of three independent yeast transformants was higher than 1,000 Miller units; (−) average β-gal activity of three independent yeast transformants was about 100 Miller units, which was similar to that of negative controls (bait plasmid alone). (B) Interaction of TRIP with TRAFs. Expression vectors encoding the NH2-terminal deletion mutants of TRAF fused to the LexA DNA binding domain were co-transformed into yeast with plasmids expressing TRIP fused to the transcription activation domain. Interactions between fusion proteins were scored by measuring β-gal activity of yeast transformants as described in Fig. 4_A._ For the analysis of the COOH-terminal deletion mutants of TRAFs, a transient transfection-based coprecipitation experiment was used. The indicated COOH-terminal deletion mutants of TRAFs were coexpressed with GST–TRIP fusion proteins in 293 cells. Cell lysates were subjected for purification with glutathione–Sepharose beads, followed by Western blot analysis with anti-TRAF1 or anti-TRAF2 polyclonal antibodies as described in Fig. 1.
Figure 5
TRAF2-mediated interaction of TRIP with CD30 or TNFR2. 293 cells were transiently transfected with the indicated combinations of equal amounts of HA-TRIP, TRAF1, TRAF2, GST-CD30, or GST-TNFR2 expression vectors for 36 h. Aliquots of cell lysates were subjected for purification with glutathione–Sepharose beads as described in Materials and Methods. Proteins coprecipitated with GST fusion proteins were analyzed by Western analysis with an anti-HA mAb (12CA5), and anti-TRAF1 or anti-TRAF2 polyclonal antibodies. In control experiments, GST proteins did not coprecipitate any of the proteins tested (data not shown). Pre, cell lysates before purification with glutathione–Sepharose beads were analyzed by Western analysis with anti-TRIP polyclonal antibodies to show that equal amounts of TRIP were expressed in each case. The positions of molecular mass markers are shown on the left. Arrows indicating the positions of TRAF1, TRAF2, or TRIP are also shown on the left.
Figure 6
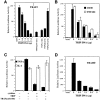
Inhibition of TRAF2-mediated NF-κB activation by TRIP overexpression. (A, left) A dose-dependent effect of TRIP expression on TRAF2-mediated NFκB activation. 293 cells were transfected with 0.5 μg of TRAF2 expression vector together with 0.5 μg of p(κB)3-IFN-LUC (28) in the presence of the indicated amount of TRIP expression vectors. Controls were transfected with 0.5 μg of pcDNA3.1 control vector and 0.5 μg of p(κB)3-IFN-LUC. All the transfections included 0.25 μg of pCMV–β-gal plasmids. 48 h after transfection, cell lysates were prepared and used for luciferase assay. All values represent luciferase activities normalized to β-gal activities and are shown as means with their respective SEMs for representative experiments performed in duplicate. (A, right) The putative coiled-coil domain of TRIP is required to inhibit TRAF2-mediated NF-κB activation. 293 cells were transfected with 0.5 μg of TRAF2 expression vector together with 0.5 μg of p(κB)3-IFN-LUC in the presence of 5 μg of plasmids expressing a dominant negative form of TRAF2 (TRAF2[241–501]), or expressing the indicated TRIP mutants. For the control experiment, cells were transfected with 0.5 μg of pcDNA3.1 control vector and 0.5 μg of p(κB)3-IFN-LUC. All the transfections included 0.25 μg of pCMV–β-gal plasmids. 48 h after transfection, cell lysates were prepared and used luciferase assay. All values represent luciferase activities normalized to β-gal activities and are shown as means with their respective SEMs for representative experiments performed in duplicate. Luciferase activity of the control experiments is shown A, left. (B) Dose-dependent inhibition of TNFR2- or CD30-mediated NF-κB activation by TRIP. 293 cells were transfected with 1 μg of plasmids expressing the chimeric receptors, CD8-TNFR2 or CD8-CD30 (30), together with 0.5 μg of p(κB)3IFN-LUC in the presence of the indicated amount of TRIP expression vectors. For the control experiment, cells were transfected with 0.5 μg of pcDNA3.1 control vector and 0.5 μg of p(κB)3-IFN-LUC. All the transfections included 0.25 μg of pCMV–β-gal plasmids. All values represent luciferase activities normalized to β-gal activities and are shown as means with their respective SEMs for representative experiments performed in duplicate. (C) TRIP overexpression inhibits TNF-induced NF-κB activation. 293 cells were transfected with 0.5 μg of p(κB)3-IFN-LUC in the presence or absence of 5 μg of plasmids expressing a dominant negative form of TRAF2 (TRAF2[241–501]), or TRIP. For the control experiment, cells were transfected with 0.5 μg of pcDNA3.1 control vector and 0.5 μg of p(κB)3-IFN-LUC. All the transfections included 0.25 μg of pCMV-β-gal plasmids. 36 h after transfection, cells were treated for 6 h with 100 pg/ml of either TNF or IL-1. All values represent luciferase activities normalized to β-gal activities and are shown as means with their respective SEMs for representative experiments performed in duplicate. (D) TRIP overexpression inhibits TRADDmediated NF-κB activation. 293 cells were transfected with 0.5 μg of plasmids expressing TRADD together with 0.5 μg of p(κB)3-IFN-LUC in the presence of the indicated amounts of TRIP expression vectors. For the control experiment, cells were transfected with 0.5 μg of pcDNA3.1 control vector and 0.5 μg of p(κB)3-IFN-LUC. All the transfections included 0.25 μg of pCMV-β-gal plasmids. All values represent luciferase activities normalized to β-gal activities and are shown as means with their respective SEMs for representative experiments performed in duplicate.
Figure 7
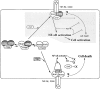
A model of interrelationship of TRAFs, c-IAP, and TRIP, and the switch of the TRAF-mediated signals between cell activation and cell death. The upper part of the diagram (shaded) describes how the receptor–TRAF signaling complex will inhibit cell death and promote cell activation/growth (7, 8, 19, 28, 29), in which A20 can work as a negative feedback regulator for TRAF2 (19). In this model, the members of the TNFR family which do not contain the death domains (e.g., TNFR2 or CD30) are postulated to trigger the induction of cell death by yet to be identified mechanism which is indicated by question mark. The lower part of the diagram explains how TRIP inhibits the TRAF-mediated cell activation/growth and contributes to the promotion of signals for cell death. For simplicity, the model does not include the receptor–TRAF2– TRAF1–TRIP complex, the presence of which cannot be excluded. However, the signals from this complex may be similar to those from the receptor–TRAF2–TRIP complex. All indicated protein association may represent dimers or higher oligomers. Three types of proximal signal transducers (TRAFs, c-IAP, or TRIP) are described. TRAFs are kept inactive in the cytoplasm due to their association with I-TRAF (also known as TANK) (8). For simplicity, a costimulatory role of I-TRAF/TANK is not included (28).
Similar articles
- Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction.
Park ES, Choi S, Shin B, Yu J, Yu J, Hwang JM, Yun H, Chung YH, Choi JS, Choi Y, Rho J. Park ES, et al. J Biol Chem. 2015 Apr 10;290(15):9660-73. doi: 10.1074/jbc.M114.609685. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716317 Free PMC article. - CD30/TNF receptor-associated factor interaction: NF-kappa B activation and binding specificity.
Lee SY, Lee SY, Kandala G, Liou ML, Liou HC, Choi Y. Lee SY, et al. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9699-703. doi: 10.1073/pnas.93.18.9699. Proc Natl Acad Sci U S A. 1996. PMID: 8790394 Free PMC article. - CD30 induction of human immunodeficiency virus gene transcription is mediated by TRAF2.
Tsitsikov EN, Wright DA, Geha RS. Tsitsikov EN, et al. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1390-5. doi: 10.1073/pnas.94.4.1390. Proc Natl Acad Sci U S A. 1997. PMID: 9037063 Free PMC article. - Tumor necrosis factor receptor-associated factors (TRAFs).
Bradley JR, Pober JS. Bradley JR, et al. Oncogene. 2001 Oct 1;20(44):6482-91. doi: 10.1038/sj.onc.1204788. Oncogene. 2001. PMID: 11607847 Review. - Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling.
Wajant H, Scheurich P. Wajant H, et al. Int J Biochem Cell Biol. 2001 Jan;33(1):19-32. doi: 10.1016/s1357-2725(00)00064-9. Int J Biochem Cell Biol. 2001. PMID: 11167129 Review.
Cited by
- The transcriptional responses of respiratory epithelial cells to Bordetella pertussis reveal host defensive and pathogen counter-defensive strategies.
Belcher CE, Drenkow J, Kehoe B, Gingeras TR, McNamara N, Lemjabbar H, Basbaum C, Relman DA. Belcher CE, et al. Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13847-52. doi: 10.1073/pnas.230262797. Proc Natl Acad Sci U S A. 2000. PMID: 11087813 Free PMC article. - The Role of Tumor Necrosis Factor Associated Factors (TRAFs) in Vascular Inflammation and Atherosclerosis.
Gissler MC, Stachon P, Wolf D, Marchini T. Gissler MC, et al. Front Cardiovasc Med. 2022 Feb 17;9:826630. doi: 10.3389/fcvm.2022.826630. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35252400 Free PMC article. Review. - Lipopolysaccharide-induced bone resorption is increased in TNF type 2 receptor-deficient mice in vivo.
Hussain Mian A, Saito H, Alles N, Shimokawa H, Aoki K, Ohya K. Hussain Mian A, et al. J Bone Miner Metab. 2008;26(5):469-77. doi: 10.1007/s00774-007-0834-0. Epub 2008 Aug 30. J Bone Miner Metab. 2008. PMID: 18758905 - TRAIP is involved in chromosome alignment and SAC regulation in mouse oocyte meiosis.
Yuan YF, Ren YX, Yuan P, Yan LY, Qiao J. Yuan YF, et al. Sci Rep. 2016 Jul 11;6:29735. doi: 10.1038/srep29735. Sci Rep. 2016. PMID: 27405720 Free PMC article. - MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling.
Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q, Kolattukudy PE, Fu M. Liang J, et al. J Exp Med. 2010 Dec 20;207(13):2959-73. doi: 10.1084/jem.20092641. Epub 2010 Nov 29. J Exp Med. 2010. PMID: 21115689 Free PMC article.
References
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. - PubMed
- Tartaglia LA, Ayres TM, Wong GHW, Goeddel DV. A novel domian within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. - PubMed
- Itoh N, Nagata S. A novel protein domain required for apoptosis. J Biol Chem. 1993;268:10932–10937. - PubMed
- Rothe M, Wong SC, Henzel WJ, Goeddel DV. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. - PubMed
- Cheng G, Cleary AM, Ye Z-S, Hong DI, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science (Wash DC) 1995;267:1494–1498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases