Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo - PubMed (original) (raw)
Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo
F Liao et al. J Exp Med. 1997.
Abstract
The inflammatory response involves sequential adhesive interactions between cell adhesion molecules of leukocytes and the endothelium. Unlike the several adhesive steps that precede it, transendothelial migration (diapedesis), the step in which leukocytes migrate between apposed endothelial cells, appears to involve primarily one adhesion molecule, platelet-endothelial cell adhesion molecule (PECAM, CD31). Therefore, we have focused on PECAM as a target for antiinflammatory therapy. We demonstrate that soluble chimeras made of the entire extracellular portion of PECAM, or of only the first immunoglobulin domain of PECAM, fused to the Fc portion of IgG, block diapedesis in vitro and in vivo. Furthermore, the truncated form of the PECAM-IgG chimera does not bind stably to its cellular ligand. This raises the possibility of selective anti-PECAM therapies that would not have the untoward opsonic or cell-activating properties of antibodies directed against PECAM.
Figures
Figure 1
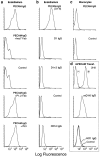
FACS® binding profiles of PECAM-IgG chimeras. Nonenzymatically resuspended HUVEC or PBMC were processed for FACScan® analysis as described in Materials and Methods. The data of this figure are arranged in vertical columns. (a) PECAM-IgG binds homophilically to PECAM on HUVEC. PECAM-IgG (100 nM) bound well to HUVEC. When binding was carried out in the presence of 100 μg/ml Fab fragments of anti-PECAM mAb hec7 (+hec7 Fab), binding was reduced to control levels seen without any primary reagent. In contrast, 100 μg/ml Fab fragments of mAb P1.2 (+P1.2 Fab), which binds to PECAM domain 6, and has been shown not to be involved in homophilic adhesion or diapedesis (4, 24), has no significant effect on PECAM-IgG binding. In addition, 7E3 (25), a blocking mAb against αvβ3, which has been reported to be a heterophilic ligand for PECAM (39, 40) did not affect PECAM-IgG binding to HUVEC (+7E3). (b) Truncated PECAM-IgG chimeras do not bind to HUVEC. While full-length PECAM-IgG (D1-6) bound well to HUVEC, chimeras consisting of domain 1 (D1 IgG) or domains 1 and 2 (D1+2 IgG) of PECAM, or a full-length CD14 chimera (CD14 IgG) did not bind significantly above background (Control). (c) When fulllength PECAM-IgG chimera (20 μg/ml, 100 nM) was incubated with monocytes under the same conditions as for HUVEC, there was no significant binding above background. (d) L cells transfected with murine PECAM (20; mPECAM Transf.) were incubated with mAb 2H8 and detected with a FITC-labeled goat anti–hamster mAb (+) or with fulllength mPECAM-IgG (mD1-6 IgG), murine domain 1-IgG (mD1 IgG), or nonbinding human IgG (Control), washed, and then incubated with FITC-labeled goat anti–human IgG. Only the mAb and full-length chimera bind to the transfectants. (d, top) −, mAb 2H8 binding to nontransfected L cells.
Figure 2
Soluble domain 1 of PECAM is sufficient to block TEM. Freshly isolated PBMC were suspended to 2 × 106/ml in medium M199 (M199), hec7 anti-PECAM mAb (20 μg/ml, 133 nM), or the indicated purified PECAM-IgG constructs at a final concentration of 100 nM. D1 IgG indicates a chimeric molecule comprised of domain 1 of human PECAM fused with the C
h
2 + C
h
3 domains of human IgG1. D1-6 IgM indicates a chimeric molecule comprised of full-length human PECAM fused with the C
h
2 + C
h
3 + C
h
4 domains of human IgM. The cells were co-cultured for 1 h at 37°C to allow transendothelial migration, and the monolayers were then washed briefly in EGTA and DPBS before fixation and quantitation of transmigration as described previously (4, 8). The data are expressed as the mean ± standard error of five replicates for each sample. All chimeric proteins containing domain 1 of PECAM significantly blocked transmigration. Asterisks (*) indicate P <0.02. The results shown are from a representative experiment of seven such experiments.
Figure 3
Domain 1-IgG blocks TEM as efficiently as full-length PECAM-IgG or mAb hec7. Freshly isolated PBMC were resuspended in the indicated concentrations of nonblocking binding control mAb W6/32 (anti–class I MHC), blocking anti-PECAM mAb hec7, or human PECAMIgG chimeras. The transmigration assay was carried out as in Fig. 2. For the mAb 133 nM is ∼20 μg/ml; for full-length PECAM-IgG, 100 nM is ∼20 μg/ml. The data shown are the mean and standard errors of three experiments with six replicates per variable in each experiment. Asterisks indicate P <0.001 compared to W6/32.
Figure 4
PECAM-IgG chimeras interfere with leukocytes, not endothelial cells in TEM. Freshly isolated PBMC were preincubated on ice for 30 min in hec7 mAb or PECAM domain 1-2 IgG or full-length PECAMIgG all at a final concentration of 100 nM (about 20 μg/ml by IgG ELISA) before being washed free of unbound reagent, resuspended to 2 × 106/ml in M199, and added to HUVEC monolayers (Preincubate with monocytes). Alternatively, the reagents were added to the apical surfaces of HUVEC monolayers for 1 h at 37°C before washing and adding untreated PBMC (Preincubate with endothelium). The third group of samples consisted of the same PBMC resuspended to the same density in the same concentrations of reagents and added together to untreated HUVEC monolayers (Added at t0). Control PBMC were resuspended in M199 alone. Transmigration proceeded for 60 min. Data were quantitated as in Fig. 2 as well as by evaluation of cross sections as described in Materials and Methods. The data shown are the mean ± standard error of six replicates for each sample. Asterisks indicate P <0.05. The experiment was performed four times with similar results.
Figure 5
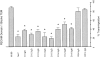
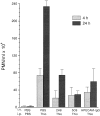
PECAM-IgG chimera blocks neutrophil emigration in vivo. Female mice of the CD2F1 strain received a single injection via tail vein (i.v.) of PBS, or 100 μg of either 2H8 anti–mouse PECAM mAb, 5C6 anti–mouse CD11b mAb, or murine PECAM-IgG chimera (mPECAMIgG) in a volume of 100 μl. 1 h later they received an intraperitoneal injection (i.p.) of 1 ml of PBS or 4% thioglycollate broth (Thio). Mice were killed at 4 h (light-shaded bars) or 24 h (dark-shaded bars) after intraperitoneal injection. Peritoneal neutrophils were collected and enumerated. The data are expressed as the mean and standard error of groups of three mice. All treatment groups are significantly different from the PBS/Thio group taken at the same time with a P value of <0.05.
Figure 6
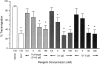
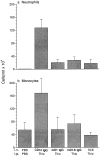
Domain 1 of PECAM blocks leukocyte emigration in vivo. Male mice of the FVB/N strain (five per group) received 100 μg of CD14-IgG chimera as a negative control, a chimeric protein consisting of domain 1 of murine PECAM (mD1 IgG), full-length murine PECAM (mD1–6 IgG), or anti-CD11b (5C6) as a positive control 1 h before thioglycollate injection. Mice were killed 18 h later and the concentration of neutrophils (a) and Mo (b) in 5 ml of peritoneal lavage fluid was determined. Data are mean and standard error. All treatment groups are significantly different from the CD14-IgG with P <0.05.
Figure 7
PECAM-IgG chimeras block leukocyte efflux at the venular lumenal surface. Representative photomicrographs from mesenteric venules of the mice in the experiment depicted in Fig. 6. (a) Venule from mouse treated with domain 1 chimera showing multiple leukocytes (some indicated by arrowheads) in apparent contact with the endothelial surface. The neutrophil at 12 o'clock is shown at higher magnification in the inset. Arrows point to endothelial cell nuclei. (b) Image from another mouse treated with domain 1 construct showing a mononuclear cell (upper) and PMN (lower) in apparent contact with the endothelial cell surface. (c) A similar image from mouse treated with full-length mPECAM-IgG with two PMN. In contrast, leukocytes were only rarely seen attached to venules of mice treated with the nonblocking CD14-IgG chimera (d) or the adhesion blocking anti-CD11b mAb 5C6 (e). Bars: (a, d, and e) 50 μm; and (b and c) 20 μm.
Figure 8
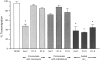
PECAM-IgG chimeras lead to increase in apparent contact of leukocytes with venule wall. The first 10 venules of appropriate size for each of the mice in the experiment of Fig. 7 were scored in a blinded fashion for leukocytes free within the lumen and those in apparent contact with the venular wall. Data are expressed as the percent of total leukocytes adherent to the wall (top) or the percent of vascular profiles with more than one attached leukocyte (bottom). The data are expressed as the mean and standard error for all mice in the group. Asterisks indicate that data are significantly different from any of the other groups with a P <0.025. Reagent, the intravenous treatment; Thio, whether or not mice received intraperitoneal injection of thioglycollate (+) or PBS (−).
Similar articles
- Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1.
Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Liao F, et al. J Exp Med. 1995 Nov 1;182(5):1337-43. doi: 10.1084/jem.182.5.1337. J Exp Med. 1995. PMID: 7595204 Free PMC article. - Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment.
Nakada MT, Amin K, Christofidou-Solomidou M, O'Brien CD, Sun J, Gurubhagavatula I, Heavner GA, Taylor AH, Paddock C, Sun QH, Zehnder JL, Newman PJ, Albelda SM, DeLisser HM. Nakada MT, et al. J Immunol. 2000 Jan 1;164(1):452-62. doi: 10.4049/jimmunol.164.1.452. J Immunol. 2000. PMID: 10605042 - The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo.
Muller WA. Muller WA. J Leukoc Biol. 1995 Apr;57(4):523-8. doi: 10.1002/jlb.57.4.523. J Leukoc Biol. 1995. PMID: 7722409 Review. - The use of anti-PECAM reagents in the control of inflammation.
Muller WA. Muller WA. Agents Actions Suppl. 1995;46:147-57. doi: 10.1007/978-3-0348-7276-8_15. Agents Actions Suppl. 1995. PMID: 7610985 Review.
Cited by
- Spatiotemporal restriction of endothelial cell calcium signaling is required during leukocyte transmigration.
Dalal PJ, Sullivan DP, Weber EW, Sacks DB, Gunzer M, Grumbach IM, Heller Brown J, Muller WA. Dalal PJ, et al. J Exp Med. 2021 Jan 4;218(1):e20192378. doi: 10.1084/jem.20192378. J Exp Med. 2021. PMID: 32970800 Free PMC article. - Neutrophil Infiltration and Function in the Pathogenesis of Inflammatory Airspace Disease.
Haynes ME, Sullivan DP, Muller WA. Haynes ME, et al. Am J Pathol. 2024 May;194(5):628-636. doi: 10.1016/j.ajpath.2023.12.008. Epub 2024 Feb 1. Am J Pathol. 2024. PMID: 38309429 Review. - Levels of soluble platelet endothelial cell adhesion molecule-1 and P-selectin are decreased in children with autism spectrum disorder.
Onore CE, Nordahl CW, Young GS, Van de Water JA, Rogers SJ, Ashwood P. Onore CE, et al. Biol Psychiatry. 2012 Dec 15;72(12):1020-5. doi: 10.1016/j.biopsych.2012.05.004. Epub 2012 Jun 19. Biol Psychiatry. 2012. PMID: 22717029 Free PMC article. - Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS.
Eugenin EA, Gamss R, Buckner C, Buono D, Klein RS, Schoenbaum EE, Calderon TM, Berman JW. Eugenin EA, et al. J Leukoc Biol. 2006 Mar;79(3):444-52. doi: 10.1189/jlb.0405215. Epub 2006 Jan 13. J Leukoc Biol. 2006. PMID: 16507710 Free PMC article. - PECAM-independent thioglycollate peritonitis is associated with a locus on murine chromosome 2.
Seidman MA, Chew TW, Schenkel AR, Muller WA. Seidman MA, et al. PLoS One. 2009;4(1):e4316. doi: 10.1371/journal.pone.0004316. Epub 2009 Jan 30. PLoS One. 2009. PMID: 19180231 Free PMC article.
References
- Carlos TM, Harlan JM. Leukocyte–endothelial cell adhesion molecules. Blood. 1994;84:2068–2101. - PubMed
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
- Muller WA. Migration of leukocytes across the vascular intima. Molecules and mechanisms. Trends Cardiovasc Med. 1995;5:15–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources