Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins - PubMed (original) (raw)
Comparative Study
Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins
L D Palmer et al. J Exp Med. 1997.
Abstract
To address the possible role of replicative senescence in human immunodeficiency virus (HIV) infection, telomere length, telomerase activity, and in vitro replicative capacity were assessed in peripheral blood T cells from HIV+ and HIV- donors. Genetic and age-specific effects on these parameters were controlled by studying HIV-discordant pairs of monozygotic twins. Telomere terminal restriction fragment (TRF) lengths from CD4+ T cells of HIV+ donors were significantly greater than those from HIV- twins. In contrast, telomere lengths in CD8+ T cells from HIV+ donors were shorter than in HIV- donors. The in vitro replicative capacity of CD4+ cells from HIV+ donors was equivalent to that of HIV- donors in response to stimulation through T cell receptor CD3 and CD28. Little or no telomerase activity was detected in freshly isolated CD4+ or CD8+ lymphocytes from HIV+ or HIV- donors, but was induced by in vitro stimulation of both HIV+ and HIV- donor cells. These results suggest that HIV infection is associated with alterations in the population dynamics of both CD4+ and CD8+ T cells, but fail to provide evidence for clonal exhaustion or replicative senescence as a mechanism underlying the decline in CD4+ T cells of HIV-infected donors.
Figures
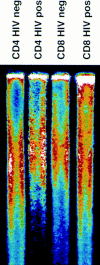
Figure 1
Telomere length in CD4+ and CD8+ T cells. Genomic DNA was isolated from purified peripheral blood CD4+ and CD8+ T cells, digested, separated by electrophoresis, and the dried and denatured gel was hybridized with a 32P end-labeled oligonucleotide (CCCTAA)3 probe. Hybridized gels were subjected to PhosphorImager analysis.
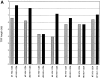
Figure 2
Telomere length in CD4+ and CD8+ T cells from HIV-positive and -negative twins. TRF analysis was carried out as described in Fig. 1, and mean TRF length calculated. (A) TRF length is shown for CD4+ T cells from HIV-negative (stippled bars) and HIV-positive (closed bars) twin donors. (B) TRF length is shown for CD8+ T cells from HIVnegative (stippled bars) and HIV-positive (closed bars) twin donors. (C) The difference in TRF length for HIV-positive and HIV-negative twin donors is shown for CD4+ (closed bar) and CD8+ (stippled bar) T cells. Positive values indicate TRF length that is greater in the HIV-positive twin, and negative values indicate TRF length that is greater in the HIV-negative twin.
Figure 2
Telomere length in CD4+ and CD8+ T cells from HIV-positive and -negative twins. TRF analysis was carried out as described in Fig. 1, and mean TRF length calculated. (A) TRF length is shown for CD4+ T cells from HIV-negative (stippled bars) and HIV-positive (closed bars) twin donors. (B) TRF length is shown for CD8+ T cells from HIVnegative (stippled bars) and HIV-positive (closed bars) twin donors. (C) The difference in TRF length for HIV-positive and HIV-negative twin donors is shown for CD4+ (closed bar) and CD8+ (stippled bar) T cells. Positive values indicate TRF length that is greater in the HIV-positive twin, and negative values indicate TRF length that is greater in the HIV-negative twin.
Figure 2
Telomere length in CD4+ and CD8+ T cells from HIV-positive and -negative twins. TRF analysis was carried out as described in Fig. 1, and mean TRF length calculated. (A) TRF length is shown for CD4+ T cells from HIV-negative (stippled bars) and HIV-positive (closed bars) twin donors. (B) TRF length is shown for CD8+ T cells from HIVnegative (stippled bars) and HIV-positive (closed bars) twin donors. (C) The difference in TRF length for HIV-positive and HIV-negative twin donors is shown for CD4+ (closed bar) and CD8+ (stippled bar) T cells. Positive values indicate TRF length that is greater in the HIV-positive twin, and negative values indicate TRF length that is greater in the HIV-negative twin.
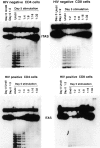
Figure 3
Telomerase activity in T cells from HIV-positive and HIVnegative donors. CD4+ and CD8+ T cells were isolated from HIV-positive and HIV-negative donors by negative selection. Telomerase activity was assayed in freshly isolated cells and after in vitro stimulation with antiCD3 and anti-CD28. T cells were stimulated for 5 d with a combination of bead-bound anti-CD3 and CD28 mAbs. Results shown are representative of 15 HIV+ donors and their HIV− controls. 293 is a transformed human kidney cell line that expresses high levels of telomerase and is used here as a positive control. HUVEC are normal epithelial cells isolated from human umbilical vein used here as a negative control.
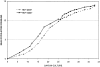
Figure 4
Replicative potential of CD4+ T cells from HIV-positive and HIV-negative donors. CD4+ T cells were repeatedly stimulated with immobilized anti-CD3 and anti-CD28. Population expansion was measured until cell cultures were unresponsive to further stimulation. The data shown are representative of five twin pairs studied.
Similar articles
- Accelerated replicative senescence of the peripheral immune system induced by HIV infection.
Bestilny LJ, Gill MJ, Mody CH, Riabowol KT. Bestilny LJ, et al. AIDS. 2000 May 5;14(7):771-80. doi: 10.1097/00002030-200005050-00002. AIDS. 2000. PMID: 10839584 - Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis.
Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Effros RB, et al. AIDS. 1996 Jul;10(8):F17-22. doi: 10.1097/00002030-199607000-00001. AIDS. 1996. PMID: 8828735 - T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover.
Wolthers KC, Bea G, Wisman A, Otto SA, de Roda Husman AM, Schaft N, de Wolf F, Goudsmit J, Coutinho RA, van der Zee AG, Meyaard L, Miedema F. Wolthers KC, et al. Science. 1996 Nov 29;274(5292):1543-7. doi: 10.1126/science.274.5292.1543. Science. 1996. PMID: 8929418 - Telomeres and HIV-1 infection: in search of exhaustion.
Wolthers KC, Miedema F. Wolthers KC, et al. Trends Microbiol. 1998 Apr;6(4):144-7. doi: 10.1016/s0966-842x(98)01233-5. Trends Microbiol. 1998. PMID: 9587191 Review. - Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection.
Bellon M, Nicot C. Bellon M, et al. Viruses. 2017 Oct 5;9(10):289. doi: 10.3390/v9100289. Viruses. 2017. PMID: 28981470 Free PMC article. Review.
Cited by
- The role of mutation accumulation in HIV progression.
Galvani AP. Galvani AP. Proc Biol Sci. 2005 Sep 7;272(1574):1851-8. doi: 10.1098/rspb.2005.3083. Proc Biol Sci. 2005. PMID: 16096099 Free PMC article. - The struggle of a good friend getting old: cellular senescence in viral responses and therapy.
Kohli J, Veenstra I, Demaria M. Kohli J, et al. EMBO Rep. 2021 Apr 7;22(4):e52243. doi: 10.15252/embr.202052243. Epub 2021 Mar 18. EMBO Rep. 2021. PMID: 33734564 Free PMC article. Review. - Cellular Senescence in Immunity against Infections.
Marrella V, Facoetti A, Cassani B. Marrella V, et al. Int J Mol Sci. 2022 Oct 6;23(19):11845. doi: 10.3390/ijms231911845. Int J Mol Sci. 2022. PMID: 36233146 Free PMC article. Review. - Single-cell analysis of lymphokine imbalance in asymptomatic HIV-1 infection: evidence for a major alteration within the CD8+ T cell subset.
Sousa AE, Victorino RM. Sousa AE, et al. Clin Exp Immunol. 1998 May;112(2):294-302. doi: 10.1046/j.1365-2249.1998.00585.x. Clin Exp Immunol. 1998. PMID: 9649194 Free PMC article. - Select neurocognitive impairment in HIV-infected women: associations with HIV viral load, hepatitis C virus, and depression, but not leukocyte telomere length.
Giesbrecht CJ, Thornton AE, Hall-Patch C, Maan EJ, Côté HC, Money DM, Murray M, Pick N. Giesbrecht CJ, et al. PLoS One. 2014 Mar 4;9(3):e89556. doi: 10.1371/journal.pone.0089556. eCollection 2014. PLoS One. 2014. PMID: 24595021 Free PMC article.
References
- Lane HC, Depper JM, Greene WC, Whalen G, Waldmann TA, Fauci AS. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;313:79–84. - PubMed
- Pantaleo G, Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. - PubMed
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Viral persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond) 1993;362:758–761. - PubMed
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature (Lond) 1990;345:458–460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials