RanBP2 associates with Ubc9p and a modified form of RanGAP1 - PubMed (original) (raw)
RanBP2 associates with Ubc9p and a modified form of RanGAP1
H Saitoh et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Ran is a small GTPase required for nuclear transport in eukaryotic cells [Gorlich, D. & Mattaj, I. W. (1996) Science 271, 1513-1518]. Mutants in Ran also show defects in mRNA processing, cell cycle regulation, and other aspects of nuclear function [Rush, M. G., Drivas, G. & D'Eustachio, P. (1996) BioEssays 18, 103-112; Sazer, S. (1996) Trends Cell Biol. 6, 81-85]. In an effort to understand the role of Ran in these diverse processes, we previously characterized 10 Ran interacting proteins (Rips) from Xenopus egg extracts. In this report, we present further characterization of a complex containing three of these Rips: p340(RanBP2), p88, and p18. We have cloned the Xenopus homologue of RanGAP1, and we show here that p88 is a modified form of this protein. In RanGAP assays, the p340(RanBP2)-p88-p18 complex contains GTPase-activating protein activity, indicating that RanGAP1 is not inactivated by modification. Rather, modification of RanGAP1 appears to be linked to its association with p340(RanBP2) because we did not observe unmodified RanGAP1 in p340(RanBP2) immunoprecipitates. We have also characterized p18, and we found that it is the Xenopus homologue of Ubc9p, an E2 ubiquitin-conjugating enzyme that is required for cell cycle regulation [Seufert, W., Futcher, B. & Jentsch, S. (1995) Nature (London) 373, 78-81]. Using antibodies directed against Xenopus Ubc9p, we have confirmed that Ubc9p associates with p340(RanBP2) in Xenopus extracts. These results suggest Ubc9p's role in cell cycle regulation may involve either modification of nuclear transport substrates or the nuclear transport machinery.
Figures
Figure 1
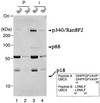
p18UBC9 and p88RanGAP1 are precipitated by anti-p340RanBP2 antibodies. Egg extract (100 μl) was incubated with preimmune serum (lanes 1 and 2) or with anti-p340RanBP2 serum, followed by precipitation with protein A-Sepharose beads. The beads were washed, and the bound proteins were eluted with 20 μl of SDS-sample buffer. Either 9 μl (lanes 1 and 3) or 3 μl (lanes 2 and 4) of eluted proteins was subjected to SDS/PAGE and Coomassie blue staining. The migration of molecular mass standards (in kDa) is indicated on the left. Arrows on the right indicate proteins that were specifically immunoprecipitated by the anti-p340RanBP2 antibodies. Two peptide sequences derived from p18 are shown as Peptides A and B, with the corresponding sequences from mammalian Ubc9p indicated below each.
Figure 2
p88 is a modified form of RanGAP1. (A) Protein sequence of Xenopus RanGAP1. The protein sequence of human RanGAP1 is shown below for comparison. Straight vertical lines indicate identical amino acids. Double dots indicate conserved residues, and single dots indicate charged residues. Gaps inserted for sequence alignment are indicated by periods. (B) p88 is a modified form of RanGAP1. Immunoprecipitations were performed from egg extracts using affinity-purified anti-RanGAP1 antibodies (I) or preimmune sera (P) from two rabbits. The precipitated proteins were analyzed by Western blotting with affinity-purified anti-RanGAP1 antibodies from one rabbit. The migration of molecular mass standards (in kDa) is indicated on the left. The two RanGAP1 bands (p88 and p65) and the IgG H and L chains are indicated on the right. (C) p340RanBP2–p88–p18 complexes contain RanGAP activity. Beads (1 μl) from an anti-p340RanBP2 immunoprecipitation (○ and •), a preimmune control incubation (▵ and ▴), or an incubation with protein A-Sepharose lacking IgG (□ and ▪) were added to GAP buffer containing 100 nM Ran–[γ-32P]GTP (○, ▵, and □) or 100 nM Ran–[α-32P]GTP (•, ▴, and ▪). At the indicated times, protein-associated 32P was measured in a filter binding assay.
Figure 4
The distribution of p340RanBP2, p88, and p18 in Xenopus egg extracts. (A) Immunoprecipitation experiments were carried out using anti-p340RanBP2 serum (lanes 2, 6, and 10) or anti-Ubc9p serum (lanes 4, 8, and 12). Control experiments were performed with corresponding guinea pig (lanes 1, 5, and 9) and rabbit (lanes 3, 7, and 11) preimmune sera. Immunoprecipitated proteins were subjected to Western blot analysis with anti-p340RanBP2 (lanes 1–4), anti-Ubc9p (lanes 5–8), or anti-RanGAP1 (lanes 9–12) serum. The positions of the 88- and 65-kDa forms of RanGAP1 are indicated to the right of lane 12. The Ig H chain detected in the Western blotting lanes 11 and 12 is indicated as IgG-H. (B) Egg extract (50 μl) was gel-filtered by Sephacryl S300 column chromatography. Fractions were analyzed by Western blotting using anti-p340RanBP2 (Top), anti-RanGAP1 (Middle), or anti-Ubc9p (Bottom) serum.
Figure 3
Cloning of Xenopus UBC9 and preparation of anti-Ubc9p antibodies. (A) Nucleotide and predicted amino acid sequence of Xenopus laevis UBC9 cDNA. The amino acid sequences obtained by peptide sequencing of p18 are underlined. The E2 active site motif, HPN(I/V)X3–4GX(I/V/L)C(I/L)X(I/V)(I/L) is boxed. (B) Xenopus egg cytosolic fraction (1 μl; lanes 1, 5, and 9), 0.1 μl of membrane faction (lanes 2, 6, and 10), or 0.1 μl of glycogen pellet fraction (lanes 3, 7, and 11) were blotted to poly(vinylidene difluoride) membranes for analysis. On the same membranes, 30 μg of total protein from Xenopus A6 tissue culture cells was also blotted (lanes 4, 8, and 12). One membrane was stained with India ink to show the transfer of proteins to the membrane (lanes 1–4). The other filters were subjected to Western blot analysis using preimmune sera (lanes 5–8) or rabbit polyclonal antiserum prepared against recombinant Xenopus Ubc9p (lanes 9–12) at a dilution of 1:5,000. The migration of molecular mass standards (in kDa) are indicated to the left in A and B. Egg interphase cytosol, membrane, and glycogen-rich fractions were prepared as described in Smythe and Newport (22). The arrow at the right indicates the position of p18.
Similar articles
- Ubc9p and the conjugation of SUMO-1 to RanGAP1 and RanBP2.
Saitoh H, Sparrow DB, Shiomi T, Pu RT, Nishimoto T, Mohun TJ, Dasso M. Saitoh H, et al. Curr Biol. 1998 Jan 15;8(2):121-4. doi: 10.1016/s0960-9822(98)70044-2. Curr Biol. 1998. PMID: 9427648 - Direct and indirect association of the small GTPase ran with nuclear pore proteins and soluble transport factors: studies in Xenopus laevis egg extracts.
Saitoh H, Cooke CA, Burgess WH, Earnshaw WC, Dasso M. Saitoh H, et al. Mol Biol Cell. 1996 Sep;7(9):1319-34. doi: 10.1091/mbc.7.9.1319. Mol Biol Cell. 1996. PMID: 8885229 Free PMC article. - A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2.
Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. Mahajan R, et al. Cell. 1997 Jan 10;88(1):97-107. doi: 10.1016/s0092-8674(00)81862-0. Cell. 1997. PMID: 9019411 - A new role of ran GTPase.
Nishimoto T. Nishimoto T. Biochem Biophys Res Commun. 1999 Sep 7;262(3):571-4. doi: 10.1006/bbrc.1999.1252. Biochem Biophys Res Commun. 1999. PMID: 10471364 Review. - Going green: plants' alternative way to position the Ran gradient.
Meier I, Xu XM, Brkljacic J, Zhao Q, Wang HJ. Meier I, et al. J Microsc. 2008 Aug;231(2):225-33. doi: 10.1111/j.1365-2818.2008.02038.x. J Microsc. 2008. PMID: 18778420 Review.
Cited by
- Phosphine-Activated Lysine Analogues for Fast Chemical Control of Protein Subcellular Localization and Protein SUMOylation.
Wesalo JS, Luo J, Morihiro K, Liu J, Deiters A. Wesalo JS, et al. Chembiochem. 2020 Jan 15;21(1-2):141-148. doi: 10.1002/cbic.201900464. Epub 2019 Oct 30. Chembiochem. 2020. PMID: 31664790 Free PMC article. - Subcellular transport of EKLF and switch-on of murine adult beta maj globin gene transcription.
Shyu YC, Lee TL, Wen SC, Chen H, Hsiao WY, Chen X, Hwang J, Shen CK. Shyu YC, et al. Mol Cell Biol. 2007 Mar;27(6):2309-23. doi: 10.1128/MCB.01875-06. Epub 2007 Jan 22. Mol Cell Biol. 2007. PMID: 17242208 Free PMC article. - Viral and cellular requirements for the nuclear entry of retroviral preintegration nucleoprotein complexes.
Matreyek KA, Engelman A. Matreyek KA, et al. Viruses. 2013 Oct 7;5(10):2483-511. doi: 10.3390/v5102483. Viruses. 2013. PMID: 24103892 Free PMC article. Review. - Nucleo-cytoplasmic partitioning of proteins in plants: implications for the regulation of environmental and developmental signalling.
Merkle T. Merkle T. Curr Genet. 2003 Dec;44(5):231-60. doi: 10.1007/s00294-003-0444-x. Epub 2003 Oct 2. Curr Genet. 2003. PMID: 14523572 Review. - Covalent modification of human immunodeficiency virus type 1 p6 by SUMO-1.
Gurer C, Berthoux L, Luban J. Gurer C, et al. J Virol. 2005 Jan;79(2):910-7. doi: 10.1128/JVI.79.2.910-917.2005. J Virol. 2005. PMID: 15613319 Free PMC article.
References
- Gorlich D, Mattaj I W. Science. 1996;271:1513–1518. - PubMed
- Rush M G, Drivas G, D’Eustachio P. BioEssays. 1996;18:103–112. - PubMed
- Sazer S. Trends Cell Biol. 1996;6:81–85. - PubMed
- Nishimoto T, Eilen E, Basilico C. Cell. 1978;15:475–483. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous