In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta - PubMed (original) (raw)
In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta
C S Hirsch et al. Proc Natl Acad Sci U S A. 1997.
Abstract
We examined the capacity of the naturally occurring inhibitors of transforming growth factor beta (TGF-beta), decorin and latency associated peptide (LAP), to reverse depressed T cell functions in peripheral blood mononuclear cells (PBMCs) from patients with pulmonary tuberculosis (TB) in vitro and to counteract the suppressive properties of TGF-beta on mycobacterial replication in blood monocytes (MN) in vitro. T cell blastogenesis in response to purified protein derivative (PPD) in PBMCs of TB patients that were cocultured with decorin or LAP reached levels comparable to those observed in healthy tuberculin-responsive control subjects. Decorin and LAP were as effective as neutralizing antibody to TGF-beta in correcting depressed T cell proliferation. Coculture of PBMCs from healthy PPD reactive individuals with neutralizing antibody to TGF-beta, decorin, or LAP did not affect T cell blastogenesis. Levels of interferon-gamma in cultures of PPD-stimulated PBMCs from patients with TB increased by more than 2-fold in the presence of maximal concentrations of either of the inhibitors of TGF-beta, whereas TGF-beta immunoreactivity declined to background levels. Coculture with optimal concentrations of decorin or LAP also led to reductions in mycobacterial growth in MN infected with Mycobacterium tuberculosis (MTB) in vitro by 51% and 62%, respectively, when compared with cells left untreated. In parallel, levels of immunoreactive TGF-beta in MTB-infected MN cultures containing decorin or LAP decreased to background levels. These data indicate that the naturally occurring inhibitors of TGF-beta, decorin and LAP, efficiently abrogate the suppressive effects of TGF-beta in PBMCs of TB patients and in MN infected with MTB in vitro. Therefore, these agents may be considered as adjuncts to antituberculous chemotherapy, and may be particularly useful in treatment of TB that is unresponsive to conventional chemotherapy.
Figures
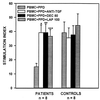
Figure 1
Effects of decorin and LAP on PPD-induced blastogenesis in TB patients. PBMCs from TB patients and control subjects were cultured for 6 days in 96-well microtiter plates in medium alone or medium containing decorin (50 μg/ml), LAP (100 ng/ml), or neutralizing antibody to TGF-β (10 μg/ml), in the absence and presence of PPD (10 μg/ml). [3H]Thymidine was present for the final 18 h of culture and incorporation of radioactivity was assessed by scintillation spectroscopy. Results are expressed as stimulation indices. ∗, P ≤ 0.0001; ∗∗, P ≤ 0.002; ∗∗∗, P ≤ 0.001, n = 8, when compared with stimulation index in PBMCs cultured in medium containing PPD alone.
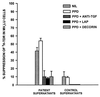
Figure 2
Effects of decorin or LAP on TGF-β bioactivity in supernatants of PBMCs from TB patients. PBMCs from TB patients and healthy tuberculin-reactive control subjects were cultured in medium alone and medium containing PPD alone or together with decorin (50 μg/ml) or LAP (100 ng/ml). Culture supernatants were obtained after 72 h and added at 50% (vol/vol) to Mv1Lu cells and incubated overnight. [3H]Thymidine was added for the final 5 h of culture and incorporation of radioactivity was assessed by scintillation spectroscopy. Results are expressed as percent suppression (mean ± SE, n = 4) of DNA synthesis in Mv1Lu cells. Decorin and LAP reversed suppression in Mv1Lu cells by supernatants of PBMCs of TB patients.
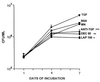
Figure 3
Effects of decorin and LAP on mycobacterial replication in MN in vitro. MN were infected with MTB (H37Ra) and cultured for up to 7 days in medium alone or medium containing optimal concentrations of antibody to TGF-β (anti-TGF-β) (10 μg/ml), decorin (DEC) (50 μg/ml), LAP (100 ng/ml), BSA (50 mg/ml), or recombinant TGF-β (10 ng/ml). Mycobacterial replication was assessed in the cfu assay. Results represent mean ± SE cfu/ml, n = 4. *, P ≤ 0.0001; **, P ≤ 0.006; ***, P ≤ 0.01 as compared with mycobacterium-infected MN cultured in medium alone (MN).
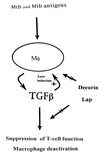
Figure 4
Schematic representation of the potential effects of the naturally occurring inhibitors of TGF-β, decorin and LAP, on TGF-β activity in situ. Presumably at sites of active MTB infection, such as tuberculous granulomas, TGF-β is produced by the mononuclear phagocytes (Mφ), which are infected with MTB and/or exposed to MTB antigens. Through positive autoregulation initiated by TGF-β itself (+), high tissue levels of the cytokine are maintained. Excess TGF-β undermines antimycobacterial defenses by suppression of T cell function and deactivation of macrophages. Decorin and LAP counteract (straight arrows) the effects of TGF-β and thereby interrupt both autoinduction and immunosuppression.
Similar articles
- Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production.
Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Hirsch CS, et al. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3193-8. doi: 10.1073/pnas.93.8.3193. Proc Natl Acad Sci U S A. 1996. PMID: 8622912 Free PMC article. - Mycobacterium tuberculosis multi-drug-resistant strain M induces IL-17+ IFNγ- CD4+ T cell expansion through an IL-23 and TGF-β-dependent mechanism in patients with MDR-TB tuberculosis.
Basile JI, Kviatcovsky D, Romero MM, Balboa L, Monteserin J, Ritacco V, Lopez B, Sabio y García C, García A, Vescovo M, Montaner PG, Palmero D, Del Carmen Sasiain M, de la Barrera S. Basile JI, et al. Clin Exp Immunol. 2017 Jan;187(1):160-173. doi: 10.1111/cei.12873. Epub 2016 Nov 2. Clin Exp Immunol. 2017. PMID: 27681197 Free PMC article. - Regulation of the human immune response during tuberculosis.
Ellner JJ. Ellner JJ. J Lab Clin Med. 1997 Nov;130(5):469-75. doi: 10.1016/s0022-2143(97)90123-2. J Lab Clin Med. 1997. PMID: 9390634 Review. - Transforming growth factor-beta: a promising target for anti-stenosis therapy.
Chamberlain J. Chamberlain J. Cardiovasc Drug Rev. 2001 Winter;19(4):329-44. doi: 10.1111/j.1527-3466.2001.tb00074.x. Cardiovasc Drug Rev. 2001. PMID: 11830751 Review.
Cited by
- T-cell recognition of Mycobacterium tuberculosis culture filtrate fractions in tuberculosis patients and their household contacts.
Demissie A, Ravn P, Olobo J, Doherty TM, Eguale T, Geletu M, Hailu W, Andersen P, Britton S. Demissie A, et al. Infect Immun. 1999 Nov;67(11):5967-71. doi: 10.1128/IAI.67.11.5967-5971.1999. Infect Immun. 1999. PMID: 10531255 Free PMC article. - A combination of a transforming growth factor-beta antagonist and an inhibitor of cyclooxygenase is an effective treatment for murine pulmonary tuberculosis.
Hernández-Pando R, Orozco-Esteves H, Maldonado HA, Aguilar-León D, Vilchis-Landeros MM, Mata-Espinosa DA, Mendoza V, López-Casillas F. Hernández-Pando R, et al. Clin Exp Immunol. 2006 May;144(2):264-72. doi: 10.1111/j.1365-2249.2006.03049.x. Clin Exp Immunol. 2006. PMID: 16634800 Free PMC article. - Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy.
Dayakar A, Chandrasekaran S, Kuchipudi SV, Kalangi SK. Dayakar A, et al. Front Immunol. 2019 Apr 5;10:670. doi: 10.3389/fimmu.2019.00670. eCollection 2019. Front Immunol. 2019. PMID: 31024534 Free PMC article. Review. - Target the Host, Kill the Bug; Targeting Host Respiratory Immunosuppressive Responses as a Novel Strategy to Improve Bacterial Clearance During Lung Infection.
Kelly AM, McLoughlin RM. Kelly AM, et al. Front Immunol. 2020 Apr 30;11:767. doi: 10.3389/fimmu.2020.00767. eCollection 2020. Front Immunol. 2020. PMID: 32425944 Free PMC article. Review. - Mycobacterium tuberculosis Induces Expansion of Foxp3 Positive CD4 T-cells with a Regulatory Profile in Tuberculin Non-sensitized Healthy Subjects: Implications for Effective Immunization against TB.
Hirsch CS, Rojas R, Wu M, Toossi Z. Hirsch CS, et al. J Clin Cell Immunol. 2016 Jun;7(3):428. doi: 10.4172/2155-9899.1000428. Epub 2016 Jun 16. J Clin Cell Immunol. 2016. PMID: 27441095 Free PMC article.
References
- Shalaby M R, Ammann A J. Cell Immunol. 1988;112:343–350. - PubMed
- Wahl S M, Hunt D A, Wong H L, Dougherty S, Mccartney-Francis N, Wahl L M, Ellingsworth L, Schmidt J A, Hall G, Roberts A B, Sporn M B. J Immunol. 1988;140:3026–3032. - PubMed
- Musso T, Espinoza-Delgado I, Pulkki K, Gusella G L, Longo D L, Varesio L. Blood. 1990;76:2466–2469. - PubMed
- Warwick-Davies J, Lowrie D B, Cole P J. J Immunol. 1995;155:3186–3193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous