Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice - PubMed (original) (raw)
Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice
M A Eglitis et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Glial cells are thought to derive embryologically from either myeloid cells of the hematopoietic system (microglia) or neuroepithelial progenitor cells (astroglia and oligodendrocytes). However, it is unclear whether the glia in adult brains free of disease or injury originate solely from cells present in the brain since the fetal stage of development, or if there is further input into such adult brains from cells originating outside the central nervous system. To test the ability of hematopoietic cells to contribute to the central nervous system, we have transplanted adult female mice with donor bone marrow cells genetically marked either with a retroviral tag or by using male donor cells. Using in situ hybridization histochemistry, a continuing influx of hematopoietic cells into the brain was detected. Marrow-derived cells were already detected in the brains of mice 3 days after transplant, and their numbers increased over the next several weeks, exceeding 14,000 cells per brain in several animals. Marrow-derived cells were widely distributed throughout the brain, including the cortex, hippocampus, thalamus, brain stem, and cerebellum. When in situ hybridization histochemistry was combined with immunohistochemical staining using lineage-specific markers, some bone marrow-derived cells were positive for the microglial antigenic marker F4/80. Other marrow-derived cells surprisingly expressed the astroglial marker glial fibrillary acidic protein. These results indicate that some microglia and astroglia arise from a precursor that is a normal constituent of adult bone marrow.
Figures
Figure 1
Detection of donor cells in the brain after bone marrow transplantation with retrovirally tagged bone marrow cells. Arrows indicate representative cells positive by ISHH with 35S-labeled oligonucleotide (A_–_C) or riboprobe (D). (A and B) Bright (A) and dark (B) field photographs of the same section. ISHH-positive cells (arrows) detected in the hippocampus of an animal 14 days postbone marrow transplantation. (C) Positive cells in the region of the septum of an animal sacrificed 14 days after bone marrow transplantation. The photograph is a double exposure of a bright field image with a dark field image of the same area. The dark field image was photographed using a red filter so that the autoradiographic grains would appear red. (D) A cell (arrow) within the ependyma of the third ventricle. [Bars = 10 μm (A_–_C) and 40 μm (D).]
Figure 2
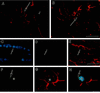
Detection of donor cells in several brain regions of a female recipient 6 weeks after transplantation with male bone marrow cells. Arrows indicate representative cells positive for the Y chromosome by ISHH. (A_–_C) Photomicrographs of a section through the ventral mesencephalon. A is photographed using a rhodamine filter to excite ethidium bromide staining of the nucleus; B is photographed using a FITC filter to excite Y chromosome-specific FITC staining; and C is photographed with a double-pass filter to show overlap of Y chromosome labeling and nucleus-specific ethidium bromide staining. Arrows indicate some of the double-labeled cells. (D_–_F) Photomicrographs demonstrating Y chromosome positive cells in other brain regions. (D) Septum. (E) Striatum. (F) Habenula. (Bars = 10 μm.)
Figure 3
Double-labeling of brain sections detects cells coexpressing the microglial marker F4/80 and the neoR retroviral tag. The F4/80 monocyte/macrophage antigen was detected by indirect immunofluorescent antibody labeling; 35S-radiolabeled probes were used to hybridize to neoR mRNA. The photomicrograph is of a representative field from an animal sacrificed 35 days after bone marrow transplantation. A cell in the center stains positive for the F4/80 antigen (red) and exhibits labeling with radioactive probe to neoR transcripts. The dark field image was photographed using a green filter so that autoradiographic grains would appear green (yellow where they overlap red immunostaining). (Bar = 10 μm.)
Figure 4
Double-labeling of brain sections detects cells coexpressing the astroglial marker GFAP and the neoR retroviral tag. (A) Detection of cells within the optic tract expressing GFAP protein using peroxidase-based immunohistochemical staining combined with ISHH to detect expression of neoR transcripts. The arrow indicates a double-labeled cell. Open arrowheads indicate clusters of grains indicative of a neoR-marked cell that is not expressing GFAP. Filled arrowheads indicate GFAP-positive positive cells that are not marked with the retroviral tag. (B and C) Detection of GFAP transcripts by ISHH using digoxigenin-labeled probes combined with detection of neoR transcripts by ISHH using 35S-labeled probes. The photograph is of a section through the cerebral cortex. (B) Polarized epifluorescent illumination to emphasize grains indicative of hybridization with 35S-labeled probe for neoR. (C) Bright field illumination emphasizing digoxigenin staining of GFAP transcripts. The cell indicated by the arrow is double-labeled. (All photomicrographs are at the same magnification. Bar = 20 μm.)
Figure 5
Male bone marrow derivative cells express GFAP. Photomicrographs are of double-labeled cells found in the brains of two different female recipient mice 10 weeks after bone marrow transplantation. Male donor cells were detected with a Y chromosome-specific riboprobe as described in Fig. 1. Astroglia were identified using a CY3-labeled polyclonal antibody against the astroglial marker GFAP. (A and B) Double-labeled cells are indicated by arrows. The arrowhead in A points to a Y chromosome-positive cell derived from the male donor marrow that is not GFAP immunopositive. A is a section through the cortex. B is a section through the septum. (Bar in B applies to A and B and equals 20 μm.) (C, D, and E) Photomicrographs of a section through the corpus callosum. In C, the section is illuminated with ultraviolet light to excite DAPI fluorescent staining of the nucleus. Nuclei from all cells are stained. D is illuminated to excite FITC staining of the Y chromosome. E is illuminated to excite CY3-immunostaining of GFAP. A Y chromosome/GFAP double labeled cell is indicated by the arrow. (Bar in E applies to C_–_E and equals 20 μm.) (F, G, and H) Photomicrographs of a single field from a section through the amygdala. F illustrates the green FITC staining associated with the Y chromosome. G shows the red GFAP immunostaining, and H is a double-exposure of the same field, first with a double band pass filter to excite FITC and CY3 fluorescence, then with ultraviolet illumination to excite the blue DAPI fluorescent staining of the nucleus (*). The green Y chromosome fluorescence is indicated by the arrow (F and H). (Bar in H applies to F_–_H and equals 4 μm.) Sections in A and C_–_E were obtained from one mouse, while those in B and F_–_H were obtained from another.
Similar articles
- Hematopoietic origin of microglial and perivascular cells in brain.
Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, Fleming PA, Drake CJ, Ogawa M. Hess DC, et al. Exp Neurol. 2004 Apr;186(2):134-44. doi: 10.1016/j.expneurol.2003.11.005. Exp Neurol. 2004. PMID: 15026252 - Bone marrow CD34+/B220+ progenitors target the inflamed brain and display in vitro differentiation potential toward microglia.
Davoust N, Vuaillat C, Cavillon G, Domenget C, Hatterer E, Bernard A, Dumontel C, Jurdic P, Malcus C, Confavreux C, Belin MF, Nataf S. Davoust N, et al. FASEB J. 2006 Oct;20(12):2081-92. doi: 10.1096/fj.05-5593com. FASEB J. 2006. PMID: 17012260 - Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia.
Simard AR, Rivest S. Simard AR, et al. FASEB J. 2004 Jun;18(9):998-1000. doi: 10.1096/fj.04-1517fje. Epub 2004 Apr 14. FASEB J. 2004. PMID: 15084516 - [The revolution of stem cells: bone marrow cells, neural cells and muscle cells are intercovertible].
Sela B. Sela B. Harefuah. 2001 Jul;140(7):628-31, 677. Harefuah. 2001. PMID: 11481968 Review. Hebrew. - Techniques and Methods of Animal Brain Surgery: Perfusion, Brain Removal, and Histological Techniques.
Soueid J, Nokkari A, Makoukji J. Soueid J, et al. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 15. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 15. PMID: 26269921 Free Books & Documents. Review.
Cited by
- The two-sided battlefield of tumour-associated macrophages in glioblastoma: unravelling their therapeutic potential.
Xiong J, Zhou X, Su L, Jiang L, Ming Z, Pang C, Fuller C, Xu K, Chi H, Zheng X. Xiong J, et al. Discov Oncol. 2024 Oct 25;15(1):590. doi: 10.1007/s12672-024-01464-5. Discov Oncol. 2024. PMID: 39453528 Free PMC article. Review. - Glial cells in the mammalian olfactory bulb.
Zhao D, Hu M, Liu S. Zhao D, et al. Front Cell Neurosci. 2024 Jul 16;18:1426094. doi: 10.3389/fncel.2024.1426094. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39081666 Free PMC article. Review. - Update on the mechanism of microglia involvement in post-stroke cognitive impairment.
Zeng T, Liu J, Zhang W, Yu Y, Ye X, Huang Q, Li P, Jiang Q. Zeng T, et al. Front Aging Neurosci. 2024 Jun 3;16:1366710. doi: 10.3389/fnagi.2024.1366710. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38887610 Free PMC article. Review. - Targeting the Multiple Complex Processes of Hypoxia-Ischemia to Achieve Neuroprotection.
Maïza A, Hamoudi R, Mabondzo A. Maïza A, et al. Int J Mol Sci. 2024 May 17;25(10):5449. doi: 10.3390/ijms25105449. Int J Mol Sci. 2024. PMID: 38791487 Free PMC article. Review. - Molecular Mechanisms in Pathophysiology of Mucopolysaccharidosis and Prospects for Innovative Therapy.
Ago Y, Rintz E, Musini KS, Ma Z, Tomatsu S. Ago Y, et al. Int J Mol Sci. 2024 Jan 17;25(2):1113. doi: 10.3390/ijms25021113. Int J Mol Sci. 2024. PMID: 38256186 Free PMC article. Review.
References
- Skoff R P, Knapp P E. In: Neuroglia. Kettenmann H, Ransom B R, editors. New York: Oxford Univ. Press; 1995. pp. 135–148.
- Theele D P, Streit W J. Glia. 1993;7:5–8. - PubMed
- Altman J. Trends Neurosci. 1994;17:47–49. - PubMed
- Lewis P D. Brain. 1968;91:721–738. - PubMed
- Kitamura T, Miyake T, Fujita S. J Comp Neurol. 1984;226:421–433. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases