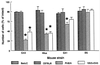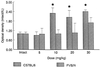Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches - PubMed (original) (raw)
Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches
P E Schauwecker et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Recent studies have sought to identify the genes involved in excitotoxic neurodegeneration. Here we report that certain strains of mice, including strains that are used for gene targeting studies, do not exhibit excitotoxic cell death after kainic acid seizures. Kainic acid produced excitotoxic cell death in the CA3 and CA1 subfields of the hippocampus in 129/SvEMS and FVB/N mice, in the same pattern as described in rats. C57BL/6 and BALB/c mice exhibited excitotoxic cell death only at very high doses of kainate, and then only in a very restricted area, although they exhibited comparable seizures. Hybrids of 129/SvEMS x C57BL/6 mice created using embryonic stem cells from 129/SvEMS mice also did not exhibit excitotoxic cell death. These results demonstrate that C57BL/6 and BALB/c strains carry gene(s) that convey protection from glutamate-induced excitotoxicity. This differential susceptibility to excitotoxicity represents a potential complication for gene targeting studies.
Figures
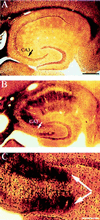
Figure 1
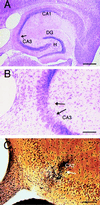
C57BL/6 mice are resistant to excitotoxin-induced neuronal cell loss and degeneration in the hippocampus. (A) Low magnification (10×) cresyl-violet staining of horizontal sections through the hippocampus in a control mouse, (B) in a FVB/N mouse 4 days post-KA injection, and (C) in a C57BL/6 mouse 4 days after KA administration (30 mg/kg). (D_–_F) Higher magnification (50×) of the CA3 subfield and the dentate hilar neurons in an uninjected control mouse, FVB/N, and C57BL/6 mouse, respectively. Note the destruction of neurons in the CA3 subfield and the dentate hilus (arrows) in FVB/N mice compared with protection against cell loss and degeneration in C57BL/6 mice. (G_–_I) High magnification (50×) of the CA3 subfield showing the extent of degeneration in an uninjected control mouse, FVB/N mouse, and C57BL/6 mouse 7 days after KA administration, respectively. Extensive degenerative debris is present throughout the CA3 subfield in FVB/N mice only. CA1 and CA3 denote the hippocampal subfields; DG, dentate gyrus. [Scale bars, 750 μm (A_–_C); 300 μm (D_–_I).]
Figure 2
Quantification of excitotoxin-induced neuronal loss in four inbred mouse strains. Differential cell loss in areas CA3, CA1, and the dentate hilus was observed among inbred mouse strains. Cell loss was most dramatic in area CA3, the dentate hilus, and area CA1 of FVB/N and 129/SvEMS inbred mouse strains, whereas no significant cell loss was observed in any areas examined in BALB/c or C57BL/6 mice (F = 6.012; P < 0.005, Tukey test).
Figure 3
In a subset of C57BL/6 mice, select cell loss and degeneration is observed in the ventral hippocampus when the dose of administered KA exceeds the LD50. (A) Low magnification (10×) cresyl-violet staining of a horizontal section through the hippocampus in a C57BL/6 mouse 7 days post-KA injection (40 mg/kg). (B) Higher magnification (66×) of the CA3 subfield showing selective cell loss in area CA3b (arrows). (C) High magnification (66×) of the CA3 subfield showing discrete degeneration within a subpopulation of cells in CA3b (arrow). CA1 and CA3 denote the hippocampal subfields; DG, dentate gyrus; H, hilus. [Scale bars, 600 μm (A) and 150 μm (B and C).]
Figure 4
Comparison of 2DG response to administration of KA in C57BL/6 and FVB/N mice. (A) High magnification (5×) of a horizontal section showing 2DG uptake in the hippocampus of a control animal. (B and C) High magnification of a horizontal section displaying 2DG uptake 60 min after injection of 20 mg/kg KA in a C57BL/6 and FVB/N mouse, respectively. Metabolism is greatest in the CA3 and CA1 subfields of the hippocampus, and hippocampal 2DG metabolism is greater in the C57BL/6 mouse (B) as compared with the FVB/N (C). [Scale bar, 750 μm.]
Figure 5
Quantitation of 2DG uptake in the hippocampus in C57BL/6 and FVB/N mice after KA administration. 2DG uptake was significantly increased in C57BL/6 mice at intermediate and high doses of KA as compared with FVB/N mice (F = 50.702; P < 0.001).
Figure 6
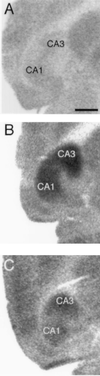
Susceptibility to excitotoxic cell death is dependent on genetic background. (A) Silver-stained section from a homozygous wild-type hybrid mouse (129/SvEMS × C57BL/6) showing a lack of degenerative debris after KA administration. (B) Selective silver-stained horizontal section from a p53−/− mouse generated in a 129/Sv background illustrating neuronal and terminal degeneration throughout the CA3 (arrows) and CA1 subfields as well as the dentate hilus 7 days after KA administration. (C) Higher magnification of area CA3 and the dentate hilus (arrows). CA3 denotes the hippocampal subfield. [Scale bars, 750 μm (A and B); 200 μm (C).]
Similar articles
- Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology.
McKhann GM 2nd, Wenzel HJ, Robbins CA, Sosunov AA, Schwartzkroin PA. McKhann GM 2nd, et al. Neuroscience. 2003;122(2):551-61. doi: 10.1016/s0306-4522(03)00562-1. Neuroscience. 2003. PMID: 14614919 - Congenic strains provide evidence that a mapped locus on chromosome 15 influences excitotoxic cell death.
Schauwecker PE. Schauwecker PE. Genes Brain Behav. 2011 Feb;10(1):100-10. doi: 10.1111/j.1601-183X.2010.00644.x. Epub 2010 Sep 29. Genes Brain Behav. 2011. PMID: 20807240 Free PMC article. - DNA damage and nonhomologous end joining in excitotoxicity: neuroprotective role of DNA-PKcs in kainic acid-induced seizures.
Neema M, Navarro-Quiroga I, Chechlacz M, Gilliams-Francis K, Liu J, Lamonica K, Lin SL, Naegele JR. Neema M, et al. Hippocampus. 2005;15(8):1057-71. doi: 10.1002/hipo.20123. Hippocampus. 2005. PMID: 16216017 - Excitotoxic neurodegeneration induced by intranasal administration of kainic acid in C57BL/6 mice.
Chen Z, Ljunggren HG, Bogdanovic N, Nennesmo I, Winblad B, Zhu J. Chen Z, et al. Brain Res. 2002 Mar 29;931(2):135-45. doi: 10.1016/s0006-8993(02)02268-0. Brain Res. 2002. PMID: 11897099 - Differences in ionotropic glutamate receptor subunit expression are not responsible for strain-dependent susceptibility to excitotoxin-induced injury.
Schauwecker PE. Schauwecker PE. Brain Res Mol Brain Res. 2003 Apr 10;112(1-2):70-81. doi: 10.1016/s0169-328x(03)00048-2. Brain Res Mol Brain Res. 2003. PMID: 12670704
Cited by
- Kainic acid-induced neuronal degeneration in hippocampal pyramidal neurons is driven by both intrinsic and extrinsic factors: analysis of FVB/N↔C57BL/6 chimeras.
Liu L, Hamre KM, Goldowitz D. Liu L, et al. J Neurosci. 2012 Aug 29;32(35):12093-101. doi: 10.1523/JNEUROSCI.6478-11.2012. J Neurosci. 2012. PMID: 22933793 Free PMC article. - Of mice and men: solving the molecular mysteries of Huntington's disease.
Shelbourne PF. Shelbourne PF. J Anat. 2000 May;196 ( Pt 4)(Pt 4):617-28. doi: 10.1046/j.1469-7580.2000.19640617.x. J Anat. 2000. PMID: 10923992 Free PMC article. Review. - A role for calcium-permeable AMPA receptors in synaptic plasticity and learning.
Wiltgen BJ, Royle GA, Gray EE, Abdipranoto A, Thangthaeng N, Jacobs N, Saab F, Tonegawa S, Heinemann SF, O'Dell TJ, Fanselow MS, Vissel B. Wiltgen BJ, et al. PLoS One. 2010 Sep 29;5(9):e12818. doi: 10.1371/journal.pone.0012818. PLoS One. 2010. PMID: 20927382 Free PMC article. - Cellular source of apolipoprotein E4 determines neuronal susceptibility to excitotoxic injury in transgenic mice.
Buttini M, Masliah E, Yu GQ, Palop JJ, Chang S, Bernardo A, Lin C, Wyss-Coray T, Huang Y, Mucke L. Buttini M, et al. Am J Pathol. 2010 Aug;177(2):563-9. doi: 10.2353/ajpath.2010.090973. Epub 2010 Jul 1. Am J Pathol. 2010. PMID: 20595630 Free PMC article. - Seizure preconditioning and epileptic tolerance: models and mechanisms.
Jimenez-Mateos EM, Henshall DC. Jimenez-Mateos EM, et al. Int J Physiol Pathophysiol Pharmacol. 2009 Nov 2;1(2):180-191. Int J Physiol Pathophysiol Pharmacol. 2009. PMID: 21383886 Free PMC article.
References
- A. A, Horrocks L A. Brain Res Rev. 1991;16:171–191. - PubMed
- Rothman S M, Olney J W. Trends Neurosci. 1987;10:299–302.
- Choi D W. Prog Brain Res. 1994;100:47–51. - PubMed
- Hori N, French-Mullen J H M, Carpenter D O. Brain Res. 1985;358:380–384. - PubMed
- Choi D W. Trends Neurosci. 1988;11:465–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous