Disaggregation of Alzheimer beta-amyloid by site-directed mAb - PubMed (original) (raw)
Disaggregation of Alzheimer beta-amyloid by site-directed mAb
B Solomon et al. Proc Natl Acad Sci U S A. 1997.
Abstract
In Alzheimer disease, beta-amyloid peptide accumulates in the brain as insoluble amyloid plaques. Amyloid filaments, similar to those found in amyloid plaques, can be assembled in vitro from chemically synthesized beta-peptides. In this study, we report that antibodies raised against the N-terminal region (1-28) of the beta-amyloid peptide bind to the in vitro-formed beta-amyloid assemblies, leading to disaggregation of the fibrils and partial restoration of the peptide's solubility. The concomitant addition of fibrillar beta-amyloid with these antibodies to PC 12 cells leads to the inhibition of the neurotoxic effects of beta-amyloid. Some of the mAbs raised against soluble beta-peptide (1-28) have been found to prevent in vitro fibrillar aggregation of beta-amyloid peptide. These experimental data suggest that site-directed mAbs interfere with the aggregation of beta-amyloid and trigger reversal to its nontoxic, normal components. The above findings give hints on how to convert in vivo senile plaques into nontoxic, diffuse components and may have therapeutic interest for those studying Alzheimer disease and other human diseases related to amyloidogenic properties of physiological peptides and proteins.
Figures
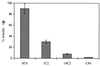
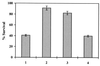
Figure 1
Solubilization of β-amyloid in the presence of mAbs 6C6, 1C2 and 14C2. The amounts of immunocomplexes of the resolubilized Aβ obtained after 24-h incubation with the respective antibodies were measured by a sandwich ELISA assay using rabbit polyclonal antibodies raised against Aβ as coating antibodies. Measurement of the OD of A495 monitors antibody binding to β-amyloid using horseradish peroxidase-labeled goat anti-mouse antibody (Bio-Rad). The data represent the mean of three replicates. The SDs of the intraassay and interassay were 8%. The percentages are related to maximal OD measured for each mAb after the addition of 100 ng of soluble Aβ complexed with each of the studied mAb to the coated ELISA wells before incubation at 37°C.
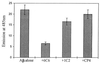
Figure 2
ThT-based fluorometric assay. Estimation of the fluorescence of ThT, which correlates with the amount of fibrillar β-amyloid formed after incubation for 1 week at 37°C before and after additional incubation for 24 h with mAbs 6C6 and 1C2 and unrelated antibodies (at molar ratio 10:1 Aβ/mAb) using the ThT assay as described.
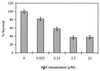
Figure 3
Dose-dependent neurotoxicity of β-amyloid aggregates on PC 12 cells measured by MTT assay. Increasing concentrations of β-amyloid aged 1 week were added to the same amount of cultured cells and assayed as described.
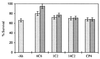
Figure 4
Selective inhibitory effect of various mAbs on the β-amyloid toxicity toward PC 12 cells using the MTT assay. The mAbs were added to 0.25 μM β-amyloid at molar ratios of 100:1 Aβ/mAb (left bars) and 10:1 Aβ/mAb (right bars), and the mixtures were added to the cells. The percentages are related to cell viability measured in the absence of the β-amyloid, which is considered 100%.
Figure 5
The effect of mAb 6C6 on the inhibition of fibrillar β-amyloid neurotoxicity toward PC12 cells using the MTT assay. The following preparations were added to the cells for 48 h: (i) Aβ (2.5 μM) preincubated for 7 days at 37°C; (ii) soluble Aβ (2.5 μM) incubated for 7 days at 37°C in the presence of mAb 6C6 (molar ratio mAb/Aβ 1:10); (iii) preformed β-amyloid (2.5 μM) incubated for a further 24 h with mAb 6C6 (molar ratio mAb/Aβ 1:10); and (iv) Aβ incubated with unrelated antibodies. The percentages are related to the cell viability measured in the absence of β-amyloid, which is considered as 100%.
Similar articles
- Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide.
Solomon B, Koppel R, Hanan E, Katzav T. Solomon B, et al. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):452-5. doi: 10.1073/pnas.93.1.452. Proc Natl Acad Sci U S A. 1996. PMID: 8552659 Free PMC article. - High affinity binding of monoclonal antibodies to the sequential epitope EFRH of beta-amyloid peptide is essential for modulation of fibrillar aggregation.
Frenkel D, Balass M, Katchalski-Katzir E, Solomon B. Frenkel D, et al. J Neuroimmunol. 1999 Mar 1;95(1-2):136-42. doi: 10.1016/s0165-5728(99)00003-x. J Neuroimmunol. 1999. PMID: 10229123 - A novel monoclonal antibody against the N-terminus of Aβ1-42 reduces plaques and improves cognition in a mouse model of Alzheimer's disease.
Xing HY, Li B, Peng D, Wang CY, Wang GY, Li P, Le YY, Wang JM, Ye G, Chen JH. Xing HY, et al. PLoS One. 2017 Jun 29;12(6):e0180076. doi: 10.1371/journal.pone.0180076. eCollection 2017. PLoS One. 2017. PMID: 28662102 Free PMC article. - Anti-aggregating antibodies, a new approach towards treatment of conformational diseases.
Solomon B. Solomon B. Curr Med Chem. 2002 Oct;9(19):1737-49. doi: 10.2174/0929867023369141. Curr Med Chem. 2002. PMID: 12369884 Review. - Beta-amyloidbased immunotherapy as a treatment of Alzheimers disease.
Solomon B. Solomon B. Drugs Today (Barc). 2007 May;43(5):333-42. doi: 10.1358/dot.2007.43.5.1062670. Drugs Today (Barc). 2007. PMID: 17724499 Review.
Cited by
- CD4 T cells in immunity and immunotherapy of Alzheimer's disease.
Monsonego A, Nemirovsky A, Harpaz I. Monsonego A, et al. Immunology. 2013 Aug;139(4):438-46. doi: 10.1111/imm.12103. Immunology. 2013. PMID: 23534386 Free PMC article. Review. - Pre-amyloid oligomers budding:a metastatic mechanism of proteotoxicity.
Bernini F, Malferrari D, Pignataro M, Bortolotti CA, Di Rocco G, Lancellotti L, Brigatti MF, Kayed R, Borsari M, Del Monte F, Castellini E. Bernini F, et al. Sci Rep. 2016 Oct 24;6:35865. doi: 10.1038/srep35865. Sci Rep. 2016. PMID: 27775057 Free PMC article. - Immunotherapeutic approaches for Alzheimer's disease.
Wisniewski T, Goñi F. Wisniewski T, et al. Neuron. 2015 Mar 18;85(6):1162-76. doi: 10.1016/j.neuron.2014.12.064. Neuron. 2015. PMID: 25789753 Free PMC article. Review. - Structural biology of cell surface receptors implicated in Alzheimer's disease.
Hermans SJ, Nero TL, Morton CJ, Gooi JH, Crespi GAN, Hancock NC, Gao C, Ishii K, Markulić J, Parker MW. Hermans SJ, et al. Biophys Rev. 2021 Nov 18;14(1):233-255. doi: 10.1007/s12551-021-00903-9. eCollection 2022 Feb. Biophys Rev. 2021. PMID: 35340615 Free PMC article. Review. - Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy.
Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Bacskai BJ, et al. J Neurosci. 2002 Sep 15;22(18):7873-8. doi: 10.1523/JNEUROSCI.22-18-07873.2002. J Neurosci. 2002. PMID: 12223540 Free PMC article.
References
- Selkoe D J. Trends Neurochem Sci. 1993;16:403–409. - PubMed
- Pike C J, Cotman C W. Brain Res. 1995;671:293–298. - PubMed
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D, Lieberburg I, Schenk D. Nature (London) 1992;359:325–327. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical