Target-independent cholinergic differentiation in the rat sympathetic nervous system - PubMed (original) (raw)
Target-independent cholinergic differentiation in the rat sympathetic nervous system
M K Schäfer et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Chemical coding in the sympathetic nervous system involves both noradrenergic and, for a minority of neurons, cholinergic neurotransmission. The expression of the cholinergic phenotype in the developing sympathetic nervous system was examined to determine if coding for cholinergic transmission occurs before or after innervation of peripheral target organs. The vesicular acetylcholine transporter (VAChT) and choline acetyltransferase, the products of the "cholinergic gene locus" determining the cholinergic phenotype, were expressed in principal cells of the paravertebral, but only rarely in prevertebral, sympathetic chains as early as embryonic day 14. A subpopulation of VAChT- and choline acetyltransferase-positive sympathetic ganglion cells persisted throughout development of the stellate and more caudal paravertebral ganglia into anatomically distinct cell groups, and into adulthood. The forepaw eccrine sweat glands, innervated exclusively by the stellate ganglion, received VAChT-positive nerve terminals at least as early as postembryonic day 4, coincident with the development of the sweat glands themselves. These terminals, like the VAChT-positive cell bodies of the developing stellate ganglion, have some noradrenergic traits including expression of tyrosine hydroxylase, but did not express the vesicular monoamine transporter, and are therefore not functionally noradrenergic. Development of the cholinergic phenotype in principal cells of the sympathetic paravertebral ganglia apparently occurs via receipt of instructive cues, or selection, within the sympathetic chain itself or perhaps even during migration of the cells of the neural crest from which the paravertebral ganglia arise.
Figures
Figure 1
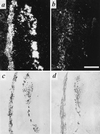
Expression of VMAT2 and VAChT in developing pre- and paravertebral ganglia of the rat. Expression of VMAT2 (a) and VAChT (b) mRNA in E14 sympathetic paravertebral (Left) and prevertebral (Right) chains detected by in situ hybridization histochemistry. Expression of VMAT2 (c) and VAChT (d) protein in E14 sympathetic paravertebral (Left) and prevertebral (Right) chains detected by immunohistochemistry. Note that the majority of principal ganglion cells in both pre- and paravertebral ganglia express VMAT2 at this stage of development. In contrast, VAChT expression is observed in several cells of the paravertebral, but only rarely in the prevertebral sympathetic chain. Note (d) the copious VAChT immunoreactivity investing principal ganglion cells of both pre- and paravertebral ganglia, and representing preganglionic cholinergic innervation of these cells, at E14. [Bar in b = 250 μm and this magnification holds for all micrographs in this figure.]
Figure 2
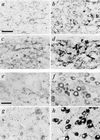
VAChT and VMAT2 expression in embryonic and adult superior cervical and stellate sympathetic ganglia. (a–d) Staining of superior cervical (a and b) and stellate (c and d) ganglia for VAChT (a and c) or VMAT2 (b and d) on E16. Note copious cholinergic preganglionic terminal immunoreactivity in both ganglia at this stage of development, large numbers of VMAT2-positive principal ganglion cells in both ganglia, and frequent VAChT-positive principal ganglion cells in stellate (c) but not superior cervical (a) ganglion at this time. (e–h) Staining of superior cervical (e and f) and stellate (g and h) ganglia for VAChT (e and g) or VMAT2 (f and h) in adult. Note copious cholinergic preganglionic terminal immunoreactivity in both ganglia, large numbers of VMAT2-positive principal ganglion cells in both ganglia, and frequent VAChT-positive principal ganglion cells in stellate (g) but not superior cervical (e) ganglion at this time. (Bars in a and e = 50 μm; all panels at same magnification.)
Figure 3
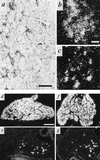
VAChT/ChAT expression in a subpopulation of principal cells of the developing stellate ganglion. (a) Immunohistochemistry for VAChT in stellate ganglion at E19. Note numerous VAChT-positive principal cells as in Fig. 2, as well as VAChT-positive preganglionic terminals at this stage of development. (b and c) Detection of VAChT mRNA (b) and ChAT mRNA (c) in E19 stellate ganglion. Exposure for 14 days following in situ hybridization. Note b and c represent nonadjacent sections from the same ganglion. (d and e) VMAT2 (d) and VAChT (e) in situ hybridization histochemistry, postnatal day 2 (P2). (f and g) VMAT2 (f) and VAChT (g) in situ hybridization histochemistry, P11. Note relative constancy in the number of VAChT mRNA-positive cells per section of whole ganglion throughout development at E19 (b), P2 (e), and P11 (g). [Bars = 50 μm (a), 10 μm (b and c), and 250 μm (d_–_g).]
Figure 4
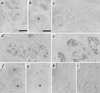
Expression of VAChT in nerve terminals innervating developing sweat glands in early postnatal development, and mutual exclusivity of VMAT2 and VAChT immunoreactivity in footpad nerve terminals. (a) Immunohistochemical staining for VAChT (as described in legend to Fig. 1) in rat sweat gland tissue at P4. Note intense VAChT-positive neuromuscular junctions within skeletal muscle at bottom left. Faint but distinct terminal staining can be seen on most of the sweat gland epithelial cell clusters, beginning to coil into sweat glands proper at P4. Nonspecific staining of blood cells is seen within lumen of large vessel separating skeletal muscle and sweat gland epithelial regions. Asterisk marks sweat gland seen at high power in b. (b) VAChT-positive terminals at P4, high magnification. Note VAChT-immunoreactivity in terminals closely contacting developing secretory coils of developing eccrine sweat glands. (c–e) VAChT-positive terminals in P8 (c), P11 (d), and adult (e) forepaw sweat glands. Note increasing density of cholinergic terminals through the first two postnatal weeks. (f–i) VAChT (f and h) and VMAT2 (g and i) in adjacent sections (f and g; h and i) of P8 sweat gland. Asterisk in f and g marks sweat gland positive for VAChT (f) and negative for VMAT2 in adjacent section (g). Arrows in h and i mark blood vessel negative for VAChT (h) and positive for VMAT2 (i). VAChT immunoreactivity in sweat gland nerve terminals was shown to be specific by complete loss of immunoreactivity upon preincubation of anti-VAChT antibody, at its final working dilution, with the C-terminal VAChT peptide against which the antibody was raised, at a final concentration of 10 μM. VMAT2 immunostaining was unaffected by preincubation with this concentration of the VAChT peptide. [Bars = 100 μm (a, c, and e) and 50 μm (b, d, and f_–_i).]
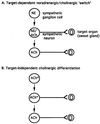
Figure 5
Models for postganglionic cholinergic neuronal differentiation in the sympathetic nervous system. (A) Sympathetic cholinergic neurons arise from principal ganglion cells via formation of fully functional noradrenergic neurons (NE) lacking cholinergic expression that synapse upon targets such as the sweat glands and induce the secretion from the target of factor(s) that induce the cholinergic phenotype and attenuate the expression of noradrenergic traits in the noradrenergic neuron (see ref. 5). (B) A proportion of the principal ganglion cells in the sympathetic chain express the cholinergic phenotype prior to innervation of target organs. Asterisk indicates a cholinergic (i.e., ChAT+/VAChT+) phenotype with noradrenergic [e.g., TH, dopamine β-hydroxylase (DBH), NET, but not VMAT] traits. Loss of noradrenergic traits in rat postganglionic cholinergic sympathetic neurons may occur as a direct result of interaction with the target, or may also be programmed in a target-independent manner.
Similar articles
- Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles.
Weihe E, Tao-Cheng JH, Schäfer MK, Erickson JD, Eiden LE. Weihe E, et al. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3547-52. doi: 10.1073/pnas.93.8.3547. Proc Natl Acad Sci U S A. 1996. PMID: 8622973 Free PMC article. - Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system.
Schäfer MK, Eiden LE, Weihe E. Schäfer MK, et al. Neuroscience. 1998 May;84(2):361-76. doi: 10.1016/s0306-4522(97)80196-0. Neuroscience. 1998. PMID: 9539210 - Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. I. Central nervous system.
Schäfer MK, Eiden LE, Weihe E. Schäfer MK, et al. Neuroscience. 1998 May;84(2):331-59. doi: 10.1016/s0306-4522(97)00516-2. Neuroscience. 1998. PMID: 9539209 - In vivo imaging of the vesicular acetylcholine transporter and the vesicular monoamine transporter.
Efange SM. Efange SM. FASEB J. 2000 Dec;14(15):2401-13. doi: 10.1096/fj.00-0204rev. FASEB J. 2000. PMID: 11099458 Review. - From the cholinergic gene locus to the cholinergic neuron.
Weihe E, Schäfer MK, Schütz B, Anlauf M, Depboylu C, Brett C, Chen L, Eiden LE. Weihe E, et al. J Physiol Paris. 1998 Oct-Dec;92(5-6):385-8. doi: 10.1016/S0928-4257(99)80010-2. J Physiol Paris. 1998. PMID: 9789842 Review.
Cited by
- Immunocytochemical properties of stellate ganglion neurons during early postnatal development.
Masliukov PM, Timmermans JP. Masliukov PM, et al. Histochem Cell Biol. 2004 Sep;122(3):201-9. doi: 10.1007/s00418-004-0692-y. Epub 2004 Aug 26. Histochem Cell Biol. 2004. PMID: 15338227 - Vesicular neurotransmitter transporters. Potential sites for the regulation of synaptic function.
Varoqui H, Erickson JD. Varoqui H, et al. Mol Neurobiol. 1997 Oct;15(2):165-91. doi: 10.1007/BF02740633. Mol Neurobiol. 1997. PMID: 9396009 Review. - Neurotrophin-3 promotes the cholinergic differentiation of sympathetic neurons.
Brodski C, Schnürch H, Dechant G. Brodski C, et al. Proc Natl Acad Sci U S A. 2000 Aug 15;97(17):9683-8. doi: 10.1073/pnas.160080697. Proc Natl Acad Sci U S A. 2000. PMID: 10931939 Free PMC article. - The sympathetic neurotransmitter switch depends on the nuclear matrix protein Satb2.
Apostolova G, Loy B, Dorn R, Dechant G. Apostolova G, et al. J Neurosci. 2010 Dec 1;30(48):16356-64. doi: 10.1523/JNEUROSCI.3502-10.2010. J Neurosci. 2010. PMID: 21123581 Free PMC article. - Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen.
Gautron L, Rutkowski JM, Burton MD, Wei W, Wan Y, Elmquist JK. Gautron L, et al. J Comp Neurol. 2013 Nov;521(16):3741-67. doi: 10.1002/cne.23376. J Comp Neurol. 2013. PMID: 23749724 Free PMC article.
References
- Groves A K, George K M, Tissier-Seta J-P, Engel J D, Brunet J-F, Anderson D J. Development (Cambridge, UK) 1995;12:887–901. - PubMed
- Reissmann E, Ernsberger U, Francis-West P H, Rueger D, Brickell P M, Rohrer H. Development (Cambridge, UK) 1996;122:2079–2088. - PubMed
- Smith J, Fauquet M, Ziller C, Le Douarin N M. Nature (London) 1979;282:853–855. - PubMed
- Landis S C, Keefe D. Dev Biol. 1983;98:349–372. - PubMed
- Landis S C. Trends Neurosci. 1990;13:344–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources