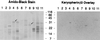Cloning and characterization of human karyopherin beta3 - PubMed (original) (raw)
Comparative Study
Cloning and characterization of human karyopherin beta3
N R Yaseen et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Nuclear import of classical nuclear localization sequence-bearing proteins is mediated by karyopherin alpha/beta1 heterodimers. A second nuclear import pathway, mediated by karyopherin beta2 (transportin), recently was described for mRNA-binding proteins. Here we report the cloning and characterization of human karyopherin beta3, which may be involved in a third pathway for nuclear import. Karyopherin beta3 was localized mainly to the cytosol and the nucleus, particularly the nuclear rim. It bound to several of the repeat-containing nucleoporins (Nup358, Nup214, Nup153, Nup98, and p62) in overlay and solution-binding assays and was competed away by karyopherin beta1. For Nup98, we localized this binding to the peptide repeat-containing region. Karyopherin beta3 contains two putative Ran-binding homology regions and bound to Ran-GTP in a solution-binding assay with much higher affinity than to Ran-GDP. Furthermore, it interacted with two ribosomal proteins in an overlay assay. We suggest that karyopherin beta3 is a nuclear transport factor that may mediate the import of some ribosomal proteins into the nucleus.
Figures
Figure 1
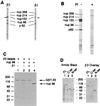
Human karyopherin β3 is homologous to yeast Kap121p and human karyopherin β1. (A) The amino acid sequence of human karyopherin β3 (hβ3) was aligned with that of Kap121p (yβ3) by the Clustal method using the Multiple Sequence Alignment module of the Lasergene software (
dnastar
). The aligned sequences were imported into SeqVu 1.1 (Garvan Institute) to highlight homologies (boxed sequences) and identities (shaded sequences); the setting used for homologies was GES scale at 85%. (B) The amino acid sequences of human karyopherin β1 and β3 were aligned as in A. (C) Comparison of Ran binding regions in RanBP1 (32), human karyopherin β1 (21) and β3; the numbers indicate amino acid positions.
Figure 2
Recombinant karyopherin β3. (A) Full-length (lane 1) and C-terminal 183 amino acids (lane 2) of karyopherin β3 were subjected to SDS/PAGE on a 5–20% acrylamide gradient gel and stained with Coomassie blue. Places of molecular mass markers are indicated to the left. (B) Fifty nanograms of recombinant karyopherin β3 (lane 1) and 40 μg of HeLa cytosol S100 fraction (lane 2) were electrophoresed, transferred to nitrocellulose, and immunoblotted with anti-karyopherin β3 antibody 384AP, and the signal was detected by chemiluminescence.
Figure 3
Immunofluorescence staining for karyopherin β3. HeLa cells treated with or without digitonin as indicated were incubated with anti-karyopherin β3 primary antibody and Cy3-conjugated secondary antibody. (Bar = 10 μm.)
Figure 4
Karyopherin β3 and karyopherin β1 compete for binding to repeat nucleoporins in overlay assay and in solution. (A) Nuclear envelope proteins were electrophoresed and transferred to nitrocellulose. Karyopherin β1 or β3 was added followed by the appropriate primary antibody and a secondary horseradish peroxidase-conjugated antibody. The signal was detected by chemiluminescence. The small arrows indicate a previously observed (3) unidentified band that interacts with both karyopherin β1 and β3. (B) Nuclear envelope protein blots were subjected to overlay assay with karyopherin β3 as in A but in the presence or absence of 10-fold molar excess of karyopherin β1 as indicated. The control lane (without karyopherin β1) contained glutathione elution buffer (see Materials and Methods) to control for the presence of this buffer in the karyopherin β1 preparation. The weak Nup98 signal in this lane may be due to the glutathione tripeptide competing with Nup98 for binding to karyopherin β3. (C) GST-karyopherin β3 fusion protein was bound to glutathione beads (lane 1), and Nup98 was added in the absence (lane 2) or presence (lane 3) of 5-fold molar excess of karyopherin β3. Lane 4 shows the Nup98 preparation in the absence of beads. (D) Bacterial lysates containing recombinant near-full length Nup98 (13) (lane 1), its C terminus (lane 2), or its repeat-containing N terminus (lane 3) were electrophoresed and transferred to nitrocellulose. The blot was stained with amido black (Left) and subjected to overlay assay with karyopherin β3 as in A (Right). Small arrows indicate the positions of the Nup98 bands in the amido black-stained gel.
Figure 5
Karyopherin β3 binds to Ran-GTP. GST-karyopherin β3 fusion protein was immobilized on beads as in Fig. 4_C_ and either Ran-GDP or Ran-GTP was added. The bound and half of the unbound fractions were electrophoresed and subjected to immunoblotting with anti-Ran antibody.
Figure 6
Karyopherin β3 binds to ribosomal proteins in an overlay assay. Ribosomal proteins were separated by reverse-phase HPLC, electrophoresed, transferred to nitrocellulose membrane, stained with amido black (Left), and subjected to overlay assay with recombinant karyopherin β3 (Right). Fractions 6–16 are shown; the remaining fractions were negative by overlay assay (not shown). Small arrows indicate the positive bands on the amido black-stained gel.
Similar articles
- The nucleoporin Nup98 is a site for GDP/GTP exchange on ran and termination of karyopherin beta 2-mediated nuclear import.
Fontoura BM, Blobel G, Yaseen NR. Fontoura BM, et al. J Biol Chem. 2000 Oct 6;275(40):31289-96. doi: 10.1074/jbc.M004651200. J Biol Chem. 2000. PMID: 10875935 - Karyopherin beta2 mediates nuclear import of a mRNA binding protein.
Bonifaci N, Moroianu J, Radu A, Blobel G. Bonifaci N, et al. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5055-60. doi: 10.1073/pnas.94.10.5055. Proc Natl Acad Sci U S A. 1997. PMID: 9144189 Free PMC article. - Structure of the nuclear transport complex karyopherin-beta2-Ran x GppNHp.
Chook YM, Blobel G. Chook YM, et al. Nature. 1999 May 20;399(6733):230-7. doi: 10.1038/20375. Nature. 1999. PMID: 10353245 - Distinct nuclear import and export pathways mediated by members of the karyopherin beta family.
Moroianu J. Moroianu J. J Cell Biochem. 1998 Aug 1;70(2):231-9. J Cell Biochem. 1998. PMID: 9671229 Review. - Molecular mechanisms of nuclear protein transport.
Moroianu J. Moroianu J. Crit Rev Eukaryot Gene Expr. 1997;7(1-2):61-72. doi: 10.1615/critreveukargeneexpr.v7.i1-2.40. Crit Rev Eukaryot Gene Expr. 1997. PMID: 9034715 Review.
Cited by
- Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells.
Jäkel S, Görlich D. Jäkel S, et al. EMBO J. 1998 Aug 3;17(15):4491-502. doi: 10.1093/emboj/17.15.4491. EMBO J. 1998. PMID: 9687515 Free PMC article. - Papillomavirus E5: the smallest oncoprotein with many functions.
Venuti A, Paolini F, Nasir L, Corteggio A, Roperto S, Campo MS, Borzacchiello G. Venuti A, et al. Mol Cancer. 2011 Nov 11;10:140. doi: 10.1186/1476-4598-10-140. Mol Cancer. 2011. PMID: 22078316 Free PMC article. Review. - Specific binding of the karyopherin Kap121p to a subunit of the nuclear pore complex containing Nup53p, Nup59p, and Nup170p.
Marelli M, Aitchison JD, Wozniak RW. Marelli M, et al. J Cell Biol. 1998 Dec 28;143(7):1813-30. doi: 10.1083/jcb.143.7.1813. J Cell Biol. 1998. PMID: 9864357 Free PMC article. - Porcine Circovirus Type 2 Hijacks Host IPO5 to Sustain the Intracytoplasmic Stability of Its Capsid Protein.
Lin C, Hu J, Dai Y, Zhang H, Xu K, Dong W, Yan Y, Peng X, Zhou J, Gu J. Lin C, et al. J Virol. 2022 Dec 14;96(23):e0152222. doi: 10.1128/jvi.01522-22. Epub 2022 Nov 21. J Virol. 2022. PMID: 36409110 Free PMC article. - Membrane orientation of the human papillomavirus type 16 E5 oncoprotein.
Krawczyk E, Suprynowicz FA, Sudarshan SR, Schlegel R. Krawczyk E, et al. J Virol. 2010 Feb;84(4):1696-703. doi: 10.1128/JVI.01968-09. Epub 2009 Dec 2. J Virol. 2010. PMID: 19955310 Free PMC article.
References
- Gorlich D, Prehn S, Laskey R A, Hartmann E. Cell. 1994;79:767–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous