Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats - PubMed (original) (raw)
Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats
I Castagliuolo et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Previously we reported that pretreatment of rats with the substance P (SP) antagonist CP-96,345 inhibits the enterotoxic responses following administration of toxin A from Clostridium difficile into ileal loops, indicating that SP participates in the intestinal responses to this toxin. We now report that injection of toxin A into rat ileum causes a rapid increase in SP content in lumbar dorsal root ganglia (DRG) and mucosal scrapings 30-60 min after toxin A administration. Toxin A-mediated fluid secretion, mannitol permeability, and ileal histologic damage is significantly increased only after 2 hr. Toxin A also causes an increase in the abundance of SP mRNA in lumbar DRG and ileal mucosa as measured by reverse transcription-PCR. Lamina propria macrophages (LPMs) obtained from toxin A-injected loops release greater amounts of tumor necrosis factor alpha (TNFalpha) and SP as compared with LPMs isolated from buffer-injected loops (P < 0.01). Pretreatment of rats with the SP antagonist CP-96,345 inhibits toxin A-mediated TNFalpha release from isolated LPMs, whereas an inactive enantiomer (CP-96,344) of the SP antagonist has no effect. LPMs obtained from toxin A-injected ileal loops incubated in vitro with SP (10(-8) to 10(-9) M) show enhanced TNFalpha secretion, whereas LPMs isolated from buffer-injected loops do not respond to SP. In addition, LPMs obtained from toxin A-injected ileal loops incubated in vitro with CP-96,345 showed a diminished TNFalpha release. Our results indicate that activated LPMs secrete SP during toxin A enteritis that can lead to secretion of cytokines, suggesting an autocrine/paracrine regulation of cytokine secretion by SP from LPMs during intestinal inflammation.
Figures
Figure 1
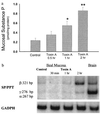
Toxin A increases SP content and SP mRNA levels in rat ileal mucosa. (a) Rat ileal loops were injected with either 5 μg of toxin A or buffer (control). At the indicated time intervals (2 hr after buffer injection) animals were killed, ileal loops were removed, and ileal mucosa was scraped and processed for measurements of SP content using an immunoenzymatic assay. Six to eight loops were tested for each time point. Results are expressed as pmol of SP per mg protein (mean ± SEM). ∗, P < 0.05; ∗∗, P < 0.01 vs. control. (b) Total RNA was purified from ileal mucosa scrapings obtained at the indicated time points. PCR of reverse transcribed cDNA was performed as described using specific primers for SP/PPT (40 cycles) or GAPDH (25 cycles). The PCR primers for SP/preprotachykinin were designed to detect the α, β, and γ forms of the PPT mRNA (36). [32P]-labeled PCR products were electrophoresed on 3.5 or 5% native polyacrylamide gels (depending on the size of the product), and autoradiograms were obtained. Arrows indicate specific SP mRNA fragments and their sizes in base pairs (bp). Negative controls performed with no RNA added to the reverse transcription, or no reverse transcriptase yielded no detectable fragments with any primer pairs.
Figure 2
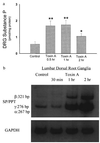
Toxin A increases SP content and SP mRNA in rat lumbar dorsal root ganglia. (a) Rat ileal loops were prepared and injected with either 5 μg toxin A or buffer (control) and animals were killed at the indicated time points (2 hr after buffer injection). Lumbar DRG from each side (n = 6–8) were removed, and SP content was measured by immunoenzymatic assay. Each bar represents the mean ± SEM of six to eight different determinations for each time point. ∗, P < 0.05; ∗∗, P < 0.01 vs. control. (b) Total RNA extracted from lumbar DRG was reverse transcribed as described. RT-PCR was performed using primers specific for SP/PPT (40 cycles) or GAPDH (25 cycles). [32P]-labeled PCR products were electrophoresed on 3.5 or 5% native polyacrylamide gels (depending on the size of the product) and autoradiograms were obtained. Arrows indicate the specific SP mRNA fragments and their sizes in bp. Negative controls were performed as described in Fig. 1_b_.
Figure 3
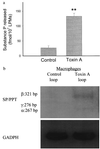
Toxin A increases release of SP and stimulates SP mRNA expression from LPMs. (a) Rat ileal loops were injected with either toxin A or buffer. After 1 hr, LPMs were purified and placed in tissue culture (6 hr at 37°C) as described. SP released into the culture medium was measured by an immunoenzymatic assay. Each bar represents the mean ± SEM of values derived from six to eight animals, each with quadruplicate determinations for each experimental condition. ∗, P < 0.01 vs. control. (b) Total RNA was extracted from LPMs after 2 hr of culture in vitro and reverse transcribed to obtain cDNA as described. SP/preprotachykinin mRNA was measured by RT-PCR as described in legend to Fig. 2_b_.
Figure 4
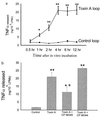
(a) Intestinal lamina propria macrophages isolated from toxin A-injected loops show enhanced release of TNFα. Ileal loops were injected with either toxin A or buffer. After 1 hr, LPMs were purified and cultured and TNFα release was determined as described. Each data point = mean ± SEM of values derived from six to eight rats, each with quadruplicate determinations for each experimental condition. ∗, P < 0.05; ∗∗, P < 0.01 vs. control. (b) Pretreatment of rats with the SP antagonist CP-96,345 reduces toxin A-induced TNFα release from LPMs. Rats were injected i.p. with either saline or saline containing CP-96,345 (2.5 mg/kg) or CP-96,344 (2.5 mg/kg) 10 min before administration of toxin A or buffer into ileal loops. After 1 hr LPMs were isolated and cultured and TNFα release after 4 hr was determined as described. Bars = mean ± SEM of values derived from six to eight rats, each with quadruplicate determinations for each experimental condition. ∗, P < 0.05; ∗∗, P < 0.01 vs. control; +, P < 0.05 vs. toxin A.
Figure 5
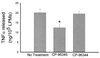
Release of TNFα from activated LPMs in vitro is inhibited by the SP antagonist CP-96,345. Rat ileal loops were injected with toxin A, and after 1 hr animals were killed, loops were excised, and intestinal LPMs were purified and plated as described. LPMs were cultured in vitro in the presence or absence of the SP receptor antagonist CP-96,345 (3 nM) or same dose of its inactive enantiomer CP-96,344. TNFα release was determined as described. Bar = mean ± SEM six different experiments, each with quadruplicate determinations per experimental condition. ∗, P < 0.05 vs. no treatment.
Similar articles
- CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin.
Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O'Keane JC, Snider RM, Leeman SE. Pothoulakis C, et al. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):947-51. doi: 10.1073/pnas.91.3.947. Proc Natl Acad Sci U S A. 1994. PMID: 7508124 Free PMC article. - CGRP upregulation in dorsal root ganglia and ileal mucosa during Clostridium difficile toxin A-induced enteritis.
Keates AC, Castagliuolo I, Qiu B, Nikulasson S, Sengupta A, Pothoulakis C. Keates AC, et al. Am J Physiol. 1998 Jan;274(1):G196-202. doi: 10.1152/ajpgi.1998.274.1.G196. Am J Physiol. 1998. PMID: 9458790 - Substance P receptor expression in intestinal epithelium in clostridium difficile toxin A enteritis in rats.
Pothoulakis C, Castagliuolo I, Leeman SE, Wang CC, Li H, Hoffman BJ, Mezey E. Pothoulakis C, et al. Am J Physiol. 1998 Jul;275(1):G68-75. doi: 10.1152/ajpgi.1998.275.1.G68. Am J Physiol. 1998. PMID: 9655686 - Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells.
Castagliuolo I, Keates AC, Wang CC, Pasha A, Valenick L, Kelly CP, Nikulasson ST, LaMont JT, Pothoulakis C. Castagliuolo I, et al. J Immunol. 1998 Jun 15;160(12):6039-45. J Immunol. 1998. PMID: 9637520 - IL-11 inhibits Clostridium difficile toxin A enterotoxicity in rat ileum.
Castagliuolo I, Kelly CP, Qiu BS, Nikulasson ST, LaMont JT, Pothoulakis C. Castagliuolo I, et al. Am J Physiol. 1997 Aug;273(2 Pt 1):G333-41. doi: 10.1152/ajpgi.1997.273.2.G333. Am J Physiol. 1997. PMID: 9277411
Cited by
- Clostridioides difficile infection drives neuronal inflammation.
Aktories K. Aktories K. Nature. 2023 Oct;622(7983):465-467. doi: 10.1038/d41586-023-02640-3. Nature. 2023. PMID: 37833474 No abstract available. - C. difficile intoxicates neurons and pericytes to drive neurogenic inflammation.
Manion J, Musser MA, Kuziel GA, Liu M, Shepherd A, Wang S, Lee PG, Zhao L, Zhang J, Marreddy RKR, Goldsmith JD, Yuan K, Hurdle JG, Gerhard R, Jin R, Rakoff-Nahoum S, Rao M, Dong M. Manion J, et al. Nature. 2023 Oct;622(7983):611-618. doi: 10.1038/s41586-023-06607-2. Epub 2023 Sep 12. Nature. 2023. PMID: 37699522 Free PMC article. - A single-cell transcriptional landscape of immune cells shows disease-specific changes of T cell and macrophage populations in human achalasia.
Liu ZQ, Dai H, Yao L, Chen WF, Wang Y, Ma LY, Li XQ, Lin SL, He MJ, Gao PT, Liu XY, Xu JX, Xu XY, Wang KH, Wang L, Chen L, Zhou PH, Li QL. Liu ZQ, et al. Nat Commun. 2023 Aug 4;14(1):4685. doi: 10.1038/s41467-023-39750-5. Nat Commun. 2023. PMID: 37542039 Free PMC article. - Gut microbiota modulates visceral sensitivity through calcitonin gene-related peptide (CGRP) production.
Pujo J, De Palma G, Lu J, Galipeau HJ, Surette MG, Collins SM, Bercik P. Pujo J, et al. Gut Microbes. 2023 Jan-Dec;15(1):2188874. doi: 10.1080/19490976.2023.2188874. Gut Microbes. 2023. PMID: 36939195 Free PMC article. - Clostridioides difficile infection: traversing host-pathogen interactions in the gut.
Cheng JKJ, Unnikrishnan M. Cheng JKJ, et al. Microbiology (Reading). 2023 Feb;169(2):001306. doi: 10.1099/mic.0.001306. Microbiology (Reading). 2023. PMID: 36848200 Free PMC article. Review.
References
- Chang M M, Leeman S E. J Biol Chem. 1970;245:4784–4790. - PubMed
- Maggi C A. Arch Int Pharmacol. 1990;303:157–166. - PubMed
- Costa M, Furness J B, Llewellyn-Smith I J. In: Physiology of the Gastrointestinal Tract. Johnson L R, editor. Vol. 1. New York: Raven; 1987. pp. 1–40.
- Keast J R, Furness J B, Costa M. J Comp Neurol. 1985;236:403–422. - PubMed
- Marchand J E, Wurm W H, Kato T, Kream R M. Pain. 1994;58:219–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous