Expression of a dominant-negative type II transforming growth factor beta (TGF-beta) receptor in the epidermis of transgenic mice blocks TGF-beta-mediated growth inhibition - PubMed (original) (raw)
Expression of a dominant-negative type II transforming growth factor beta (TGF-beta) receptor in the epidermis of transgenic mice blocks TGF-beta-mediated growth inhibition
X J Wang et al. Proc Natl Acad Sci U S A. 1997.
Abstract
To determine whether a functional type II receptor of transforming growth factor beta (TGF-beta) is required to mediate the growth inhibitory effect of TGF-beta on the skin in vivo, we have generated transgenic mice that overexpress a dominant negative-type II TGF-beta receptor (delta beta RII) in the epidermis. The delta beta RII mice exhibited a thickened and wrinkled skin, and histologically the epidermis was markedly hyperplastic and hyperkeratotic. In vivo labeling with BrdUrd showed a 2.5-fold increase in the labeling index over controls, with labeled nuclei occurring in both basal and suprabasal cells of transgenic epidermis. In heterozygotes, this skin phenotype gradually diminished, and by 10-14 days after birth the transgenic mice were indistinguishable from their normal siblings. However, when F1 mice were mated to homozygosity, perinatal lethality occurred due to the severe hyperkeratotic phenotype, which restricted movement. Cultured primary keratinocytes from delta beta RII mice also exhibited an increased rate of growth in comparison with nontransgenic controls, and were resistant to TGF-beta-induced growth inhibition. These data document the role of the type II TGF-beta receptor in mediating TGF-beta-induced growth inhibition of the epidermis in vivo and in maintenance of epidermal homeostasis.
Figures
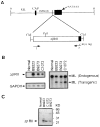
Figure 1
Transgene construct and expression. (A) Schematic showing structure of the ML.ΔβRII transgene. Construction and characterization of the ML targeting vector have been described (19). The ΔβRII was produced by PCR, which retained the entire extracellular domain and the transmembrane domain, but deletes most of the cytoplasmic domain (8). A sequence tag, encoding the human c-myc epitope (11), was introduced to the 3′ end of the transgene. This transgene was then inserted into the _Cla_I site of the ML vector. Primers 1 and 2, specific for the TGF-β type II receptor and human c-myc tag, respectively, were used for PCR to screen transgenic mice. (B) RNase protection assays to identify ML.ΔβRII transgene expression. Note that the transgene expression detected by the ΔβRII probe was exclusively in transgenic epidermis (Left), and the ratio of the signal intensity between ΔβRII and GAPDH was 4:1 for RNA from line B9273, and 5:1 for RNAs from transgenic lines B9223 and C1072. The ML probe was used to detect both the endogenous loricrin and transgene expression (Right). The intensity of ΔβRII expression versus endogenous loricrin was 0.4:1 from transgenic line B9273 and 0.6:1 from lines B9223 and C1072. (C) Western blotting showing ΔβRII protein expression. A 23-kDa band is detected in transgenic epidermis from all lines, but not in the normal control, and this represents the size predicated for the truncated ΔβRII protein. Epidermal extracts from lines C1072 and B9223 exhibited a higher intensity than line B9273, which was consistent with RNA expression levels (see B). Nonspecific bands, resulting from the rabbit anti-mouse secondary antibody, were seen in a similar pattern in all of the lanes. The B9223 extract gave a slightly higher background due to partial degradation.
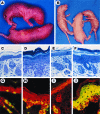
Figure 2
ML.ΔβRII gross phenotype, histopathology, BrdUrd labeling, and K6 expression. (A) A transgenic pup (Upper) from line B9223 shows a thickened and wrinkled skin compared with the nontransgenic sibling (Lower) 24 hr after birth. (B) Transgenic mice (Left and Right) from line B9223 exhibited a peeling skin phenotype 3 days after birth. (C_–_F) Richardson’s staining of 1 μm sections. (×20.) (C) Nontransgenic skin 24 hr after birth. (D) ML.ΔβRII transgenic skin from line B9223 shows a 3-fold increase in thickness of the epidermis, hyperkeratosis, and hypertrophic keratinocytes compared with the control (C). (E) Nontransgenic skin 3 days after birth. (F) ML.ΔβRII transgenic skin from line B9223 shows a moderate hyperplasia at 3 days, and a more loosely attached stratum corneum compared with the transgenic skin at 24 hr (D). (G_–_J) Double-label immunofluorescence. (×20.) (Red represents K14.) (G) BrdUrd labeling (green or yellow) in a nontransgenic epidermis. (H) ML.ΔβRII transgenic epidermis exhibits about a 3-fold increase in BrdUrd labeling. (I) K6 is only expressed in the hair follicles of the normal skin (green or yellow). (J) K6 overexpression in ML.ΔβRII transgenic epidermis.
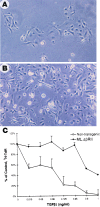
Figure 3
Analysis of ML.ΔβRII keratinocytes in culture. (A) Nontransgenic keratinocytes were subconfluent at day 4 of culture. (B) Keratinocytes from ML.ΔβRII epidermis grew faster and were confluent by day 4. (C) [3H]TdR labeling of keratinocytes 22 hr after addition of TGF-β1. [3H]TdR labeling for cultures receiving different concentrations of TGF-β1 was expressed as percent of control. Each point is the average of results determined in triplicate. Error bars represent SD. Note that nontransgenic keratinocytes showed a marked decrease in [3H]TdR labeling at the lowest concentration of TGF-β1, while ML.ΔβRII keratinocytes were resistant to TGF-β1 until the concentration reached 0.5 ng/ml.
Similar articles
- Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis.
Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, Wang XJ. Han G, et al. J Clin Invest. 2005 Jul;115(7):1714-23. doi: 10.1172/JCI24399. Epub 2005 Jun 2. J Clin Invest. 2005. PMID: 15937546 Free PMC article. - Concerted action of TGF-beta 1 and its type II receptor in control of epidermal homeostasis in transgenic mice.
Cui W, Fowlis DJ, Cousins FM, Duffie E, Bryson S, Balmain A, Akhurst RJ. Cui W, et al. Genes Dev. 1995 Apr 15;9(8):945-55. doi: 10.1101/gad.9.8.945. Genes Dev. 1995. PMID: 7774812 - TGF beta regulation of cell proliferation.
Moses HL, Arteaga CL, Alexandrow MG, Dagnino L, Kawabata M, Pierce DF Jr, Serra R. Moses HL, et al. Princess Takamatsu Symp. 1994;24:250-63. Princess Takamatsu Symp. 1994. PMID: 8983080 Review. - Regulation of epithelial proliferation by TGF-beta.
Moses HL, Yang EY, Pietenpol JA. Moses HL, et al. Ciba Found Symp. 1991;157:66-74; discussion 75-80. doi: 10.1002/9780470514061.ch5. Ciba Found Symp. 1991. PMID: 2070684 Review.
Cited by
- Dual Role of alpha6beta4 integrin in epidermal tumor growth: tumor-suppressive versus tumor-promoting function.
Raymond K, Kreft M, Song JY, Janssen H, Sonnenberg A. Raymond K, et al. Mol Biol Cell. 2007 Nov;18(11):4210-21. doi: 10.1091/mbc.e06-08-0720. Epub 2007 Aug 15. Mol Biol Cell. 2007. PMID: 17699601 Free PMC article. - Overexpression of a kinase-deficient transforming growth factor-beta type II receptor in mouse mammary stroma results in increased epithelial branching.
Joseph H, Gorska AE, Sohn P, Moses HL, Serra R. Joseph H, et al. Mol Biol Cell. 1999 Apr;10(4):1221-34. doi: 10.1091/mbc.10.4.1221. Mol Biol Cell. 1999. PMID: 10198068 Free PMC article. - Infrequently methylated event at sites -362 to -142 in the promoter of TGF beta R1 gene in non-small cell lung cancer.
Zhao J, Liu Z, Li W, Liu X, Chen XF, Zhang HT. Zhao J, et al. J Cancer Res Clin Oncol. 2008 Aug;134(8):919-25. doi: 10.1007/s00432-008-0392-4. Epub 2008 Apr 18. J Cancer Res Clin Oncol. 2008. PMID: 18421475 - Deficiency in Neuronal TGF-β Signaling Leads to Nigrostriatal Degeneration and Activation of TGF-β Signaling Protects against MPTP Neurotoxicity in Mice.
Tesseur I, Nguyen A, Chang B, Li L, Woodling NS, Wyss-Coray T, Luo J. Tesseur I, et al. J Neurosci. 2017 Apr 26;37(17):4584-4592. doi: 10.1523/JNEUROSCI.2952-16.2017. Epub 2017 Mar 31. J Neurosci. 2017. PMID: 28363982 Free PMC article. - Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis.
Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, Wang XJ. Han G, et al. J Clin Invest. 2005 Jul;115(7):1714-23. doi: 10.1172/JCI24399. Epub 2005 Jun 2. J Clin Invest. 2005. PMID: 15937546 Free PMC article.
References
- Roberts A B, Flanders K C, Kondaiah P, Thompson N L, Obberghen-Schilling E V, Wakefield L, Rossi P, Crombrugghe B D, Heine U, Sporn M B. Recent Prog Horm Res. 1988;44:157–193. - PubMed
- Pittelkow M R, Coffey R J, Moses H L. Ann NY Acad Sci. 1988;548:211–224. - PubMed
- Cui W, Fowlis D J, Cousins F M, Duffie E, Bryson S, Balmain A, Akhurst R J. Genes Dev. 1995;9:945–955. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases