Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression - PubMed (original) (raw)
Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression
Y Ilan et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Recombinant adenoviruses (Ads) are highly efficient at transferring foreign genes to the liver in vivo; however, the duration of gene expression is limited by the host antiviral immune response, which prevents expression upon readministration of the virus. To test whether overexpression of the immunomodulatory products of the early Ad genome region 3 (E3) could prevent the antiviral immune response and prolong expression of foreign genes delivered by Ad vectors, we injected a recombinant Ad (Ad-E3-hBUGT), containing both E3 and the human bilirubin-uridine-diphosphoglucuronate-glucuronosyltransferase (BUGT) genes, into BUGT-deficient hyperbilirubinemic Gunn rats. Control Gunn rats received Ad-hBUGT, which expresses human BUGT alone. An initial injection of either virus resulted in hepatic expression of human BUGT as evidenced by excretion of bilirubin glucuronides in bile and a reduction of mean serum bilirubin levels from 7.0 mg/dl to 1.9-2.7 mg/dl within 7 days. In Ad-E3-hBUGT-injected rats, serum bilirubin levels increased to 4.5 mg/dl by 84 days after infection, but a second administration of the virus on that day resulted in a hypobilirubinemic response similar to that seen with the first injection. In contrast, rats receiving Ad-hBUGT had serum bilirubin levels of 7 mg/dl on day 84 after infection, but showed no reduction of serum bilirubin by reinjection of the virus on that day. In the rats injected with Ad-E3-hBUGT, but not in the ones injected with Ad-hBUGT, there was a marked inhibition of the antiviral antibody and Ad-specific cytotoxic T lymphocyte responses. This is the first demonstration that insertion of E3 genes in recombinant Ads facilitates readministration of a functional vector for long-term correction of an inherited metabolic disorder.
Figures
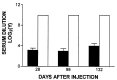
Figure 1
Anti-Ad neutralizing antibody levels in Ad-E3-hBUGT- and Ad-hBUGT-treated rats. Antibody levels were measured in sera from nine rats in Group A (Ad-E3-hBUGT, solid bars) and seven control rats from Group C (Ad-hBUGT, open bars), 28 days after the first virus injection. In Group A, four animals had no detectable titers in undiluted sera but were included in the calculations of the average and the standard deviation. In Group C, all animals had titers greater than/or equal to 1:1024, which was the highest dilution of serum tested. Corresponding values are shown for day 98 (eight animals in Group A, four with undetectable titers; five animals in Group C) and for day 132 (eight animals in Group A, four with undetectable titers; seven animals in Group C).
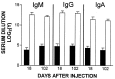
Figure 2
Anti-Ad ELISA levels in Ad-E3-hBUGT- and Ad-hBUGT-treated rats. Antibody levels (IgM, IgG, and IgA) were measured in sera from 4–5 rats in Group A (Ad-E3-hBUGT, solid bars) and 5–6 control rats from Group C (Ad-hBUGT, open bars) on day 18, after the first virus injection and on day 102, after the second virus injection. Averages and standard deviations for all animals are shown for each group. (There was a single animal in Group A that had no detectable IgM, IgG, and IgA antibodies on day 18, and a different Group A animal that lacked all three antibodies on day 102).
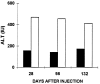
Figure 3
Effect of Ad-E3-hBUGT tolerization on CTLs. CTLs were assayed in Ad-E3-hBUGT-treated rats (Group A, solid bars) and control Ad-hBUGT-treated rats (Group C, open bars). T cells were harvested on days 28, 98, and 132 from two rats in each group and were used as effector cells against Ad-infected primary hepatocytes. CTL killing was expressed as ALT levels released from Ad-infected hepatocytes as described.
Figure 4
The presence of the hBUGT gene in rat liver after the second administration of Ad-E3-hBUGT virus. Liver specimens, from rats in Groups A and C were obtained 7 days after the second recombinant virus injection (day 91 of the experiment), and tested by PCR for the presence of the 321-bp band of hBUGT DNA (see Materials and Methods). Positive control, normal human liver (H); negative control, untreated Gunn rat (G).
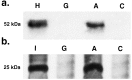
Figure 5
(a) Expression of hBUGT protein on day 91 in liver homogenates from a representative rat in Group A compared with a rat from Group C. The positive control was normal human liver (H); the negative control was untreated Gunn rat (G). A 52-kDa band is seen only in the liver of the rat from Group A. (b) Expression of the gp19K-E3 protein in liver from a rat in Group A compared with a rat from Group C. The positive control was 293 cells transfected with the gp19K (I); the negative control was a liver specimen from an untreated Gunn rat (G). A 25-kDa band representing the glycosylated size of gp19K is seen in the specimen from Group A but not Group C.
Figure 6
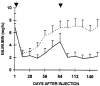
Effect of E3 expression on serum bilirubin levels in rats from Group A (solid line) that received two Ad-E3-hBUGT injections (days 1 and 84). Control rats (broken line) from Group C received Ad-hBUGT injections on the same days.
Figure 7
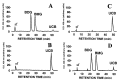
HPLC analysis of bile pigments. Bile was collected by cannulation of the bile ducts of rats from Groups A and C, 21 days after the second virus injection. (A) A normal Wistar rat bile analysis profile where greater than 95% of the bilirubin in the bile is conjugated. (B) Bile analysis of an untreated Gunn rat in which all of the bilirubin in bile is unconjugated. (C) Bile analysis from a control rat from Group C after the second virus injection. The vast majority of the bilirubin is unconjugated. (D) Analysis of bile in a rat from Group A after the second injection showing that most of the bilirubin is conjugated. BMG, bilirubin monoglucuronide; BDG, bilirubin diglucuronide; UCB, unconjugated bilirubin.
Similar articles
- Oral tolerization to adenoviral proteins permits repeated adenovirus-mediated gene therapy in rats with pre-existing immunity to adenoviruses.
Ilan Y, Sauter B, Chowdhury NR, Reddy BV, Thummala NR, Droguett G, Davidson A, Ott M, Horwitz MS, Chowdhury JR. Ilan Y, et al. Hepatology. 1998 May;27(5):1368-76. doi: 10.1002/hep.510270525. Hepatology. 1998. PMID: 9581693 - A non-immunogenic adenoviral vector, coexpressing CTLA4Ig and bilirubin-uridine-diphosphoglucuronateglucuronosyltransferase permits long-term, repeatable transgene expression in the Gunn rat model of Crigler-Najjar syndrome.
Thummala NR, Ghosh SS, Lee SW, Reddy B, Davidson A, Horwitz MS, Chowdhury JR, Chowdhury NR. Thummala NR, et al. Gene Ther. 2002 Aug;9(15):981-90. doi: 10.1038/sj.gt.3301729. Gene Ther. 2002. PMID: 12101428 - Transient immunosuppression with FK506 permits long-term expression of therapeutic genes introduced into the liver using recombinant adenoviruses in the rat.
Ilan Y, Jona VK, Sengupta K, Davidson A, Horwitz MS, Roy-Chowdhury N, Roy-Chowdhury J. Ilan Y, et al. Hepatology. 1997 Oct;26(4):949-56. doi: 10.1002/hep.510260422. Hepatology. 1997. PMID: 9328318 - Subversion of host defense mechanisms by adenoviruses.
Burgert HG, Ruzsics Z, Obermeier S, Hilgendorf A, Windheim M, Elsing A. Burgert HG, et al. Curr Top Microbiol Immunol. 2002;269:273-318. doi: 10.1007/978-3-642-59421-2_16. Curr Top Microbiol Immunol. 2002. PMID: 12224514 Review. - Function of adenovirus E3 proteins and their interactions with immunoregulatory cell proteins.
Horwitz MS. Horwitz MS. J Gene Med. 2004 Feb;6 Suppl 1:S172-83. doi: 10.1002/jgm.495. J Gene Med. 2004. PMID: 14978760 Review.
Cited by
- A novel hybrid adenoretroviral vector with more extensive E3 deletion extends transgene expression in submandibular glands.
Zheng C, Cotrim AP, Nikolov N, Mineshiba F, Swaim W, Baum BJ. Zheng C, et al. Hum Gene Ther Methods. 2012 Jun;23(3):169-81. doi: 10.1089/hgtb.2011.175. Epub 2012 Jul 20. Hum Gene Ther Methods. 2012. PMID: 22817829 Free PMC article. - Adenovirus-mediated persistent cystic fibrosis transmembrane conductance regulator expression in mouse airway epithelium.
Scaria A, St George JA, Jiang C, Kaplan JM, Wadsworth SC, Gregory RJ. Scaria A, et al. J Virol. 1998 Sep;72(9):7302-9. doi: 10.1128/JVI.72.9.7302-7309.1998. J Virol. 1998. PMID: 9696826 Free PMC article. - Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted.
Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Amalfitano A, et al. J Virol. 1998 Feb;72(2):926-33. doi: 10.1128/JVI.72.2.926-933.1998. J Virol. 1998. PMID: 9444984 Free PMC article. - Improving adenovirus based gene transfer: strategies to accomplish immune evasion.
Seregin SS, Amalfitano A. Seregin SS, et al. Viruses. 2010 Sep;2(9):2013-2036. doi: 10.3390/v2092013. Epub 2010 Sep 24. Viruses. 2010. PMID: 21994718 Free PMC article. - Inhibition of tumor necrosis factor alpha-induced NF-kappa B activation by the adenovirus E3-10.4/14.5K complex.
Friedman JM, Horwitz MS. Friedman JM, et al. J Virol. 2002 Jun;76(11):5515-21. doi: 10.1128/jvi.76.11.5515-5521.2002. J Virol. 2002. PMID: 11991979 Free PMC article.
References
- Ali M, Lemoine N R, Ring C J A. Gene Ther. 1994;1:367–384. - PubMed
- Jaffe H A, Danel C, Longenecker G. Nat Genet. 1992;1:372–378. - PubMed
- Prevec L, Schneider M, Rosenthal K L, Belbeck L W, Derbyshire J B, Graham F L. J Gen Virol. 1989;70:429–434. - PubMed
- Graham F L, Prevec L. In: Methods in Molecular Biology. Murray E J, editor. Clifton, NJ: Humana; 1991. pp. 109–128. - PubMed
- Horwitz M S. In: Virology. Fields B N, Knipe D M, editors. New York: Raven; 1990. pp. 1679–1721.
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK046057/DK/NIDDK NIH HHS/United States
- R01 DK039137/DK/NIDDK NIH HHS/United States
- P30 DK041296/DK/NIDDK NIH HHS/United States
- R01-DK 46057/DK/NIDDK NIH HHS/United States
- T32 DK007218/DK/NIDDK NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- R01-DK 39137/DK/NIDDK NIH HHS/United States
- P30-DK 41296/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical