Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses - PubMed (original) (raw)
Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses
S Choi et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Changes in synaptic efficacy are crucial for the development of appropriate neural circuits and brain information storage. We have investigated mechanisms underlying long-term depression (LTD) at glutamatergic synapses in the striatum, a brain region important in motor performance and cognition, and a target for Huntington and Parkinson diseases. Induction of striatal LTD is dependent on postsynaptic depolarization and calcium influx through L-type channels. Surprisingly, LTD maintenance appears to involve a decrease in the probability of neurotransmitter release from presynaptic terminals as evidenced by increases in paired-pulse facilitation and the coefficient of variation of synaptic responses that are tightly associated with LTD expression. Furthermore, both the apparent probability of neurotransmitter release and the magnitude of LTD decrease concomitantly during postnatal development, consistent with the idea that striatal LTD is involved in a developmental decrease in the probability of neurotransmitter release at corticostriatal synapses. The presynaptic changes that underlie striatal LTD may also be important for motor performance and certain forms of learning and memory.
Figures
Figure 1
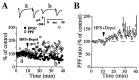
Dependence of striatal LTD induction on postsynaptic depolarization (Depol). (A) LTD was induced by stimulating corticostriatal afferents at 100 Hz for 1 sec along with simultaneous 1-sec depolarization to −10 or 0 mV, repeated four times at 10-sec intervals (n = 11). The pipette contained a standard intracellular solution. (B) HFS alone in voltage-clamp condition at −80 or −90 mV failed to induce LTD (n = 5). (C) Depolarization to 0 mV (1-sec duration, repeated four times at 10-sec intervals) in the absence of HFS failed to induce LTD (n = 8). Points in each graph are values averaged over 1-min time epochs. EPSCs shown above the graph are recorded at times indicated by letters. Waveforms in A_–_C are averages of 20 individual EPSCs.
Figure 2
Changes in PPF ratio and CV values induced by manipulations that alter presynaptic but not postsynaptic function at corticostriatal synapses. (A) Decreasing Ca2+/Mg2+ ratio in the external solution increased both the PPF ratio and CV value (n = 4). PPF ratio after decreasing Ca2+/Mg2+ ratio in the external solution was 141.1 ± 12.7% of control (two-tailed repeated measures t test, P < 0.05). (_B_) Partial blockade of AMPA/kainate receptors did not alter either the PPF ratio or the CV value (_n_ = 4). PPF ratio after partially blocking AMPA/kainate receptors was 106 ± 2.7% of control (_P_ > 0.1). (C) Decreasing stimulus (Stim) strength increased CV values, but did not change PPF (n = 6). PPF ratio after decreasing stimulus strength was 102.8 ± 4.7% (P > 0.5). (D) Summary of effects of pre- or postsynaptic modulations on average peak EPSC amplitudes and CV values. The CV value after decreasing Ca2+/Mg2+ ratio, partially blocking AMPA/kainate receptors and decreasing stimulus intensities, was 132 ± 6.1, 97.5 ± 3.8, and 175.2 ± 10.9 of control, respectively (P < 0.05, _P_ > 0.5, and P < 0.005, respectively). Points in A_–_C are values averaged over 1-min time epochs. EPSCs shown above the graph are recorded at times indicated by letters. Waveforms in A_–_C are averages of 20 individual paired EPSCs. DNQX, 6,7-dinitroquinoxaline-2,3(1H,4H)-dione.
Figure 3
Striatal LTD is closely associated with increases in PPF ratio. (A Upper) Paired EPSCs recorded at the indicated times before and after LTD induction. (Lower) EPSC peak amplitude and PPF ratio from the same neuron plotted against time before and after LTD induction. (B) Summary graph showing increases in PPF ratio after LTD induction (n = 11). PPF ratio after LTD induction was 137.9 ± 7.3% of control. Note that the change in PPF ratio associated with LTD expression is similar to that observed after decreasing Ca2+/Mg2+ ratio in the external solution (see Fig. 2_A_). Waveforms in A are averages of 20 individual paired EPSCs. Points in B are values averaged over 1-min time epochs.
Figure 4
Increases in PPF ratio are strongly associated with striatal LTD. (A) Effect of 10 μM nifedipine on LTD induction and PPF ratio (n = 6). (B) Effect of 10 mM EGTA applied in the intracellular solution on LTD induction and PPF ratio (n = 5). (C and D, Upper) Paired EPSCs taken at the indicated times before and after either HFS or depolarization (Depol) alone, respectively. (Lower) PPF ratio plotted against time before and after HFS alone (C, n = 5) or depolarization (D, n = 8). Waveforms in C and D are averages of 20 individual paired EPSCs. Points in A_–_D are values averaged over 1-min time epochs.
Figure 5
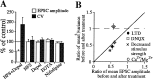
The increase in CV associated with striatal LTD is sufficient to explain the magnitude of synaptic depression. (A) Summary of results using different protocols comparing changes in average peak EPSC amplitudes and CV values. (B) Graph summarizing the effects on μ2/σ2 (1/CV2) of all of the manipulations that affected synaptic strength. These include DNQX (n = 4), decreased stimulus intensity (n = 6), decreased Ca2+/Mg2+ ratio (n = 4), and LTD (n = 11). If the mechanism of LTD expression is due predominantly to the change in Pr or n then values either on the diagonal line or below would be expected, whereas, if changes in postsynaptic response to transmitter account for LTD expression then no change in μ2/σ2 would be expected. Depol, depolarization.
Figure 6
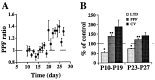
A developmental increase in basal PPF ratio is associated with a decrease in the susceptibility to striatal LTD. (A) Basal PPF ratio plotted as a function of age (n = 171 cells). PPF ratio measured in P10–P15 group (n = 68) and P23–P27 group (n = 42) averaged 0.97 ± 0.02 and 1.29 ± 0.03, respectively (unpaired t test, P < 0.0001). There was no significant difference between the two age groups in average peak EPSC amplitude of the first pulse of the paired responses. The range of stimulus intensity used to evoke the response and series resistance of whole-cell patch-clamp recordings did not differ across age. (B) Summary of bar graph comparing changes induced by LTD induction in average peak EPSC amplitudes, PPF ratio, and CV values in P10–P19 group (n = 11) and P23–P27 group (n = 13). The difference in the magnitude of LTD and the increase in PPF after LTD induction between the two age groups was statistically significant (two-tailed, unpaired t test; ∗, P < 0.005; ∗∗, P < 0.05). There was no significant difference in the increase in CV after LTD induction between the two groups. PPF ratio and CV value after LTD induction in the P23–P27 group were 119.3 ± 3.8% and 139.9 ± 15.9% of control, respectively (P < 0.0005 and P < 0.05, respectively). Note that we failed to induce LTD in 4 out of 17 slices in the P23–P27 group and only the values from the 13 slices showing LTD are included in the graph. Basal PPF ratio in the neurons from the P10–P19 and P23–P27 groups in which LTD was examined averaged 1.017 ± 0.054 and 1.255 ± 0.056, respectively (two-tailed, unpaired test, P < 0.01).
Similar articles
- Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat.
Ronesi J, Lovinger DM. Ronesi J, et al. J Physiol. 2005 Jan 1;562(Pt 1):245-56. doi: 10.1113/jphysiol.2004.068460. Epub 2004 Oct 21. J Physiol. 2005. PMID: 15498813 Free PMC article. - Bi-directional changes in synaptic plasticity induced at corticostriatal synapses in vitro.
Spencer JP, Murphy KP. Spencer JP, et al. Exp Brain Res. 2000 Dec;135(4):497-503. doi: 10.1007/s002210000523. Exp Brain Res. 2000. PMID: 11156313 - Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum.
Partridge JG, Tang KC, Lovinger DM. Partridge JG, et al. J Neurophysiol. 2000 Sep;84(3):1422-9. doi: 10.1152/jn.2000.84.3.1422. J Neurophysiol. 2000. PMID: 10980015 - Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum.
Lovinger DM. Lovinger DM. Neuropharmacology. 2010 Jun;58(7):951-61. doi: 10.1016/j.neuropharm.2010.01.008. Epub 2010 Jan 21. Neuropharmacology. 2010. PMID: 20096294 Free PMC article. Review. - Presynaptic LTP and LTD of excitatory and inhibitory synapses.
Castillo PE. Castillo PE. Cold Spring Harb Perspect Biol. 2012 Feb 1;4(2):a005728. doi: 10.1101/cshperspect.a005728. Cold Spring Harb Perspect Biol. 2012. PMID: 22147943 Free PMC article. Review.
Cited by
- The two faces of synaptic failure in AppNL-G-F knock-in mice.
Latif-Hernandez A, Sabanov V, Ahmed T, Craessaerts K, Saito T, Saido T, Balschun D. Latif-Hernandez A, et al. Alzheimers Res Ther. 2020 Aug 24;12(1):100. doi: 10.1186/s13195-020-00667-6. Alzheimers Res Ther. 2020. PMID: 32838792 Free PMC article. - Developmental regulation of thalamus-driven pauses in striatal cholinergic interneurons.
McGuirt A, Pigulevskiy I, Sulzer D. McGuirt A, et al. iScience. 2022 Oct 13;25(11):105332. doi: 10.1016/j.isci.2022.105332. eCollection 2022 Nov 18. iScience. 2022. PMID: 36325074 Free PMC article. - Spiny projection neurons exhibit transcriptional signatures within subregions of the dorsal striatum.
Roman KM, Dinasarapu AR, VanSchoiack A, Ross PM, Kroeppler D, Jinnah HA, Hess EJ. Roman KM, et al. Cell Rep. 2023 Nov 28;42(11):113435. doi: 10.1016/j.celrep.2023.113435. Epub 2023 Nov 11. Cell Rep. 2023. PMID: 37952158 Free PMC article. - Cue-conditioned alcohol seeking in rats following abstinence: involvement of metabotropic glutamate 5 receptors.
Adams CL, Short JL, Lawrence AJ. Adams CL, et al. Br J Pharmacol. 2010 Feb 1;159(3):534-42. doi: 10.1111/j.1476-5381.2009.00562.x. Epub 2010 Jan 8. Br J Pharmacol. 2010. PMID: 20067474 Free PMC article. - Parallel decrease in omega-conotoxin-sensitive transmission and dopamine-induced inhibition at the striatal synapse of developing rats.
Momiyama T. Momiyama T. J Physiol. 2003 Jan 15;546(Pt 2):483-90. doi: 10.1113/jphysiol.2002.031773. J Physiol. 2003. PMID: 12527734 Free PMC article.
References
- Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. - PubMed
- Ito M. Annu Rev Neurosci. 1989;12:85–102. - PubMed
- Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. - PubMed
- Kullmann D M, Siegelbaum S A. Neuron. 1995;15:997–1002. - PubMed
- Stevens C F, Wang Y. Nature (London) 1994;371:704–707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical