Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation - PubMed (original) (raw)
Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation
P J Goulder et al. J Exp Med. 1997.
Abstract
Primary human immunodeficiency virus (HIV) infection is controlled principally by HIV-specific cytotoxic T lymphocytes (CTL) to a steady-state level of virus load, which strongly influences the ultimate rate of progression to disease. Epitope selection by CTL may be an important determinant of the degree of immune control over the virus. This report describes the CTL responses of two HLA-identical hemophiliac brothers who were exposed to identical batches of Factor VIII and became seropositive within 10 wk of one another. Both have HLA-A*0201. The CTL responses of the two siblings were very dissimilar, one donor making strong responses to two epitopes within p17 Gag (HLA-A*0201-restricted SLYNTVATL and HLA-A3-restricted RLRPGGKKK). The sibling responded to neither epitope, but made strong responses to two epitopes presented by HLA-B7. This was not the result of differences in presentation of the epitopes. However, mutations in both immunodominant epitopes of the p17 Gag responder were seen in proviral sequences of the nonresponder. We then documented the CTL responses to two HLA-A*0201-restricted epitopes, in Gag (SLYNTVATL) and Pol (ILKEPVHGV) in 22 other HIV-infected donors with HLA-A*0201. The majority (71%) generated responses to the Gag epitope. In the 29% of donors failing to respond to the Gag epitope in standard assays, there was evidence of low frequency memory CTL responses using peptide stimulation of PBMC, and most of these donors also showed mutations in or around the Gag epitope. We concluded that HLA class I genotype determines epitope selection initially but that mutation in immunodominant epitopes can profoundly alter the pattern of CTL response.
Figures
Figure 1
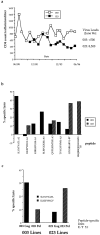
(a) Serial CD4 counts and latest viral loads in donors 003 and 023. (b) Pattern of recognition of CTL epitopes in bulk cultured lymphocytes from donors 003 and 023. E/T ratio, 50:1. Only peptides recognized by lymphocytes from 003 or 023 are shown. HLA-A*0201–restricted epitopes, SLYNTVATL and ILKEPVHGV; HLA-A3–restricted epitopes, KIRLRPGGK, QVPLRPMTYK, TVYGVPVWK (reference 17), and RLRPGGKKK; HLA-B7–restricted epitopes, GPGHKARVL and HSQRRQDILDLWIY (Goulder, P.J.R., unpublished data). Lysis of targets pulsed with no peptide subtracted to calculate percent specific lysis. Assay timepoint, January, 1996; very similar responses were observed from timepoint July, 1996. (c) Peptide stimulation of PBMC allows detection of Pol-specific CTL in donor 003 and Gag-specific CTL in donor 023. Assay timepoint, June, 1994; very similar responses were observed from the December, 1994 timepoint.
Figure 2
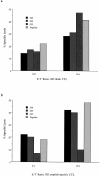
(a) Bulk cultured CTL from donor 003 recognized Gag–vaccinia-infected BCL targets from donor 023 (who made no HLA-A2– or HLA-A3–restricted Gag response; HLA-identical) and from donor 5M (a Gag responder; HLA matched through HLA-A*0201 and HLA-B51). E/T ratio, 18:1 and 76:1 as shown. Recognition of targets pulsed with 10 μm SLYNTVATL also is shown. Lysis of targets pulsed with no peptide or infected with control PB2–vaccinia subtracted to calculate percent specific lysis. In separate assays, BCL from Pol responders 023, 065, and 008 similarly presented the SLYNTVATL epitope to Gag-specific effectors from donors 102, 077, and 868 (data not shown). (b) HLA-A*0201– restricted ILKEPVHGV (Pol)-specific line from donor 023 recognized Pol–vaccinia-infected BCL targets from two Gag responders, 003 (HLAidentical) and 5M (matched through HLA-A*0201 and HLA-B51). Recognition of BCL targets pulsed with 10 μm ILKEPVHGV also is shown. In separate assays, BCL from Gag responders 46M and 868 similarly presented the ILKEPVHGV epitope to Pol-specific effectors from donors 241, 46M, and 868 (data not shown).
Figure 3
(a) Recognition of SLYNTVATL variants by a SLYNTVATL-specific line from donor 868; E/T 3:1. Lysis of targets pulsed with no peptide subtracted to calculate percent specific lysis. This assay was repeated on three occasions with the same pattern of peptide recognition (data not shown). Bulk-cultured lymphocytes from donor 003 also showed no recognition of the SLHNAVAVL variant (0% specific lysis) compared with recognition of SLYNTVATL (45% specific lysis) (data not shown). (b) Recognition of the HLA-A3–restricted peptide epitope RLRPGGKKK by bulk-cultured CTL from donor 003, and the variant RLRPGGKKC (encoded by proviral DNA within donor 023); E/T ratios as shown. Peptide concentrations, 50 μM. Peptide titration assays were also performed using a peptide-specific line from donor 003: the variant peptide RLRPGGKKC was not recognized at peptide concentrations of below 50 μM, whereas the index peptide was recognized above peptide concentrations of 5 nM (data not shown). (c) Recognition of defined HLA-A*0201–, HLA-B8–, and HLA-B62–restricted CTL epitopes (references and 38) by bulk-cultured CTL from donor 46M (HLA class I -A and -B tissue type: HLA-A1/2 -B8/62) at two timepoints: March, 1995, and December, 1996. Bulk CTL cultured for 15–16 d in each case before assay. E/T ratio, 100:1. Peptide concentrations: 10 μM. A2 Gag, SLYNTVATL. A2 Pol, ILKEPVHGV. B8 17.3, GGKKKYKL. B8 17.8, ELRSLYNTV. B8 24.13, EIYKRWII. B8 24.20, DCKTILKAL. B8 Pol, GPKVKQWPL. B8 Nef 1, WPTVRERM. B8 Nef 8, FLKEKGGL. B62 p24, GLNKIVRMY. B62 Pol, ILKEPVHGVY. B62 Nef, TQGYFPDWQNY. (d) SLYNTVATL-specific killing is not detectable in peptide-specific lines stimulated using SLFNTVATL, but is generated using the index peptide to stimulate PBMC from donor 46M. E/T ratios: SLFNTVATL line, 14:1; SLYNTVATL line, 6:1. Lysis of targets pulsed with no peptide subtracted to calculate percent specific lysis. Assay performed at day 16 of culture; the same result was observed at day 23 of culture (data not shown).
Similar articles
- Absence of immunodominant anti-Gag p17 (SL9) responses among Gag CTL-positive, HIV-uninfected vaccine recipients expressing the HLA-A*0201 allele.
Ferrari G, Neal W, Ottinger J, Jones AM, Edwards BH, Goepfert P, Betts MR, Koup RA, Buchbinder S, McElrath MJ, Tartaglia J, Weinhold KJ. Ferrari G, et al. J Immunol. 2004 Aug 1;173(3):2126-33. doi: 10.4049/jimmunol.173.3.2126. J Immunol. 2004. PMID: 15265949 - Recognition patterns of HLA-A2-restricted human immunodeficiency virus-1-specific cytotoxic T-lymphocytes in a cohort of HIV-1-infected individuals.
Schmitt-Haendle M, Bachmann O, Harrer E, Schmidt B, Bäuerle M, Harrer T. Schmitt-Haendle M, et al. Viral Immunol. 2005;18(4):627-36. doi: 10.1089/vim.2005.18.627. Viral Immunol. 2005. PMID: 16359229 - An HLA-directed molecular and bioinformatics approach identifies new HLA-A11 HIV-1 subtype E cytotoxic T lymphocyte epitopes in HIV-1-infected Thais.
Bond KB, Sriwanthana B, Hodge TW, De Groot AS, Mastro TD, Young NL, Promadej N, Altman JD, Limpakarnjanarat K, McNicholl JM. Bond KB, et al. AIDS Res Hum Retroviruses. 2001 May 20;17(8):703-17. doi: 10.1089/088922201750236988. AIDS Res Hum Retroviruses. 2001. PMID: 11429111 - Identification of highly conserved and broadly cross-reactive HIV type 1 cytotoxic T lymphocyte epitopes as candidate immunogens for inclusion in Mycobacterium bovis BCG-vectored HIV vaccines.
Ferrari G, Kostyu DD, Cox J, Dawson DV, Flores J, Weinhold KJ, Osmanov S. Ferrari G, et al. AIDS Res Hum Retroviruses. 2000 Sep 20;16(14):1433-43. doi: 10.1089/08892220050140982. AIDS Res Hum Retroviruses. 2000. PMID: 11018863 Review. - Rapid characterization of HIV clade C-specific cytotoxic T lymphocyte responses in infected African children and adults.
Goulder PJ. Goulder PJ. Ann N Y Acad Sci. 2000 Nov;918:330-45. doi: 10.1111/j.1749-6632.2000.tb05502.x. Ann N Y Acad Sci. 2000. PMID: 11131720 Review.
Cited by
- An integrative bioinformatic approach for studying escape mutations in human immunodeficiency virus type 1 gag in the Pumwani Sex Worker Cohort.
Peters HO, Mendoza MG, Capina RE, Luo M, Mao X, Gubbins M, Nagelkerke NJ, Macarthur I, Sheardown BB, Kimani J, Wachihi C, Thavaneswaran S, Plummer FA. Peters HO, et al. J Virol. 2008 Feb;82(4):1980-92. doi: 10.1128/JVI.02742-06. Epub 2007 Dec 5. J Virol. 2008. PMID: 18057233 Free PMC article. - Systematic identification of immunodominant CD4+ T cell responses to HpaA in Helicobacter pylori infected individuals.
Hu J, Chen L, Yang W, Li B, Sun H, Wei S, He Y, Zhao Z, Yang S, Zou Q, Chen W, Guo H, Wu C. Hu J, et al. Oncotarget. 2016 Aug 23;7(34):54380-54391. doi: 10.18632/oncotarget.11092. Oncotarget. 2016. PMID: 27509059 Free PMC article. - Constraints on HIV-1 evolution and immunodominance revealed in monozygotic adult twins infected with the same virus.
Draenert R, Allen TM, Liu Y, Wrin T, Chappey C, Verrill CL, Sirera G, Eldridge RL, Lahaie MP, Ruiz L, Clotet B, Petropoulos CJ, Walker BD, Martinez-Picado J. Draenert R, et al. J Exp Med. 2006 Mar 20;203(3):529-39. doi: 10.1084/jem.20052116. Epub 2006 Mar 13. J Exp Med. 2006. PMID: 16533886 Free PMC article. - Recognition of HIV-1 peptides by host CTL is related to HIV-1 similarity to human proteins.
Rolland M, Nickle DC, Deng W, Frahm N, Brander C, Learn GH, Heckerman D, Jojic N, Jojic V, Walker BD, Mullins JI. Rolland M, et al. PLoS One. 2007 Sep 5;2(9):e823. doi: 10.1371/journal.pone.0000823. PLoS One. 2007. PMID: 17786195 Free PMC article. - Memory CD8+ T cells in HIV infection.
McMichael AJ, Ogg G, Wilson J, Callan M, Hambleton S, Appay V, Kelleher T, Rowland-Jones S. McMichael AJ, et al. Philos Trans R Soc Lond B Biol Sci. 2000 Mar 29;355(1395):363-7. doi: 10.1098/rstb.2000.0575. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10794056 Free PMC article. Review.
References
- Yap K, Ada G, McKenzie I. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza. Nature (Lond) 1978;273:238–239. - PubMed
- Riddell SR, Watanabe KS, Goodrich JM, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of CTL clones. Science (Wash DC) 1992;257:238–241. - PubMed
- Heslop HE, Ng CYC, Li C, Smith CA, Loftin SK, Krance RA, Brenner MK, Rooney CM. Longterm restoration of immunity against Epstein–Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials