The dynamic nuclear redistribution of an hnRNP K-homologous protein during Drosophila embryo development and heat shock. Flexibility of transcription sites in vivo - PubMed (original) (raw)
The dynamic nuclear redistribution of an hnRNP K-homologous protein during Drosophila embryo development and heat shock. Flexibility of transcription sites in vivo
P Buchenau et al. J Cell Biol. 1997.
Abstract
The Drosophila protein Hrb57A has sequence homology to mammalian heterogenous nuclear ribonucleoprotein (hnRNP) K proteins. Its in vivo distribution has been studied at high resolution by confocal laser scanning microscopy (CLSM) in embryos injected with fluorescently labeled monoclonal antibody. Injection of antibody into living embryos had no apparent deleterious effects on further development. Furthermore, the antibody-protein complex could be observed for more than 7 cell cycles in vivo, revealing a dynamic redistribution from the nucleus to cytoplasm at each mitosis from blastoderm until hatching. The evaluation of two- and three-dimensional CLSM data sets demonstrated important differences in the localization of the protein in the nuclei of living compared to fixed embryos. The Hrb57A protein was recruited to the 93D locus upon heat shock and thus serves as an in vivo probe for the activity of the gene in diploid cells of the embryo. Observations during heat shock revealed considerable mobility within interphase nuclei of this transcription site. Furthermore, the reinitiation as well as the down regulation of transcriptional loci in vivo during the recovery from heat shock could be followed by the rapid redistribution of the hnRNP K during stress recovery. These data are incompatible with a model of the interphase nucleus in which transcription complexes are associated with a rigid nuclear matrix.
Figures
Figure 2
Nuclear import and mitotic distribution of Hrb57A in living embryos. A shows selected frames from a time series of confocal images from a single blastoderm embryo during the nuclear cycles 11–14. The cycle number is given at the bottom of each panel. M denotes mitosis. Arrows indicate the nonfluorescent mitotic chromatin (first panel) and a weak accumulation of Hrb57A in cycle 11 interphase nuclei (second panel). (B) Plot of the mean nuclear fluorescence intensity measured in a single focal plane for a series of images measured at 1 min intervals during cycle 13. An import of Hrb57A is evident for the 10 min during interphase while the sharp decline of fluorescence falls together with nuclear division. C and D are time series of confocal images showing nuclear division in blastoderm (C) and after gastrulation (D). I, interphase; M, metaphase; A, anaphase. Arrows denote the location of the dividing chromatin which contains a detectable amount of Hrb57A after (D) but not before (C) gastrulation. Bar, 10 μm.
Figure 2
Nuclear import and mitotic distribution of Hrb57A in living embryos. A shows selected frames from a time series of confocal images from a single blastoderm embryo during the nuclear cycles 11–14. The cycle number is given at the bottom of each panel. M denotes mitosis. Arrows indicate the nonfluorescent mitotic chromatin (first panel) and a weak accumulation of Hrb57A in cycle 11 interphase nuclei (second panel). (B) Plot of the mean nuclear fluorescence intensity measured in a single focal plane for a series of images measured at 1 min intervals during cycle 13. An import of Hrb57A is evident for the 10 min during interphase while the sharp decline of fluorescence falls together with nuclear division. C and D are time series of confocal images showing nuclear division in blastoderm (C) and after gastrulation (D). I, interphase; M, metaphase; A, anaphase. Arrows denote the location of the dividing chromatin which contains a detectable amount of Hrb57A after (D) but not before (C) gastrulation. Bar, 10 μm.
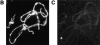
Figure 7
Detection and mobility of the 93D locus in vivo. Living embryos were injected with rhodaminecoupled mAb Q18. After development at room temperature, embryos were heat shocked at 37°C directly on the stage of the microscope to mark the 93D locus. (A) A field of epidermal interphase nuclei from an embryo 75 min after the beginning of heat shock. (B) Time series of 2-D projections of a single amnioserosa nucleus from a heat-shocked embryo during germ band elongation. The original data set consisted of seven 1-μm Z-axis sections per time point. The schematic drawing in C shows the location of the nucleolus (N) and the 93D loci (a–d) within the nucleus in B. (D) A plot of the relative minimal distances between the loci of the nucleus in B against time. The distances are given relative to the first image of the series. (E) 10 two-body distance plots against time demonstrating the time-dependent changes in the distances between 93D loci during heat shock. The distances were measured from 2-D projections of image stacks from seven different interphase nuclei in several embryos.
Figure 7
Detection and mobility of the 93D locus in vivo. Living embryos were injected with rhodaminecoupled mAb Q18. After development at room temperature, embryos were heat shocked at 37°C directly on the stage of the microscope to mark the 93D locus. (A) A field of epidermal interphase nuclei from an embryo 75 min after the beginning of heat shock. (B) Time series of 2-D projections of a single amnioserosa nucleus from a heat-shocked embryo during germ band elongation. The original data set consisted of seven 1-μm Z-axis sections per time point. The schematic drawing in C shows the location of the nucleolus (N) and the 93D loci (a–d) within the nucleus in B. (D) A plot of the relative minimal distances between the loci of the nucleus in B against time. The distances are given relative to the first image of the series. (E) 10 two-body distance plots against time demonstrating the time-dependent changes in the distances between 93D loci during heat shock. The distances were measured from 2-D projections of image stacks from seven different interphase nuclei in several embryos.
Figure 7
Detection and mobility of the 93D locus in vivo. Living embryos were injected with rhodaminecoupled mAb Q18. After development at room temperature, embryos were heat shocked at 37°C directly on the stage of the microscope to mark the 93D locus. (A) A field of epidermal interphase nuclei from an embryo 75 min after the beginning of heat shock. (B) Time series of 2-D projections of a single amnioserosa nucleus from a heat-shocked embryo during germ band elongation. The original data set consisted of seven 1-μm Z-axis sections per time point. The schematic drawing in C shows the location of the nucleolus (N) and the 93D loci (a–d) within the nucleus in B. (D) A plot of the relative minimal distances between the loci of the nucleus in B against time. The distances are given relative to the first image of the series. (E) 10 two-body distance plots against time demonstrating the time-dependent changes in the distances between 93D loci during heat shock. The distances were measured from 2-D projections of image stacks from seven different interphase nuclei in several embryos.
Figure 7
Detection and mobility of the 93D locus in vivo. Living embryos were injected with rhodaminecoupled mAb Q18. After development at room temperature, embryos were heat shocked at 37°C directly on the stage of the microscope to mark the 93D locus. (A) A field of epidermal interphase nuclei from an embryo 75 min after the beginning of heat shock. (B) Time series of 2-D projections of a single amnioserosa nucleus from a heat-shocked embryo during germ band elongation. The original data set consisted of seven 1-μm Z-axis sections per time point. The schematic drawing in C shows the location of the nucleolus (N) and the 93D loci (a–d) within the nucleus in B. (D) A plot of the relative minimal distances between the loci of the nucleus in B against time. The distances are given relative to the first image of the series. (E) 10 two-body distance plots against time demonstrating the time-dependent changes in the distances between 93D loci during heat shock. The distances were measured from 2-D projections of image stacks from seven different interphase nuclei in several embryos.
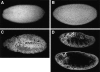
Figure 1
The distribution of Hrb57A in fixed and living embryos. The overall distribution of Hrb57A during embryogenesis was observed in fixed embryos (A–C) using indirect antibody staining or followed directly in vivo by microinjection of rhodaminecoupled mAb Q18 into living embryos (D). (A) Preferential cytoplasmic localization of Hrb57A, syncytial blastoderm, nuclear cycle 13. (B) Nuclear localization, cellular blastoderm, cycle 14. (C) Hrb57A is nuclear in all tissues during later embryogenesis, as shown here for an embryo of stage 10, at the time of full germ band elongation. (D) The similar complete localization of Hrb57A in late embryogenesis occurs in the microinjected embryos. Two optical sections through a living embryo of stage 12 during germ band retraction, showing the in vivo labeling pattern of Hrb57A. All images are single confocal sections.
Figure 3
The 3-D intranuclear distribution of Hrb57A in situ and in vivo. (A) Stereo image showing the distribution in discrete loci of the Hrb57A protein in a fixed interphase nucleus of a wholemount embryo reconstructed from 25 optical sections with a Z axis distance of 0.25 μm. (B) Stereo image of a part of a similar nucleus in a living embryo, reconstructed from 6 optical sections separated by 0.4 μm. Both image stacks have been recorded with a Plan-neofluar oil immersion objective 63×, NA 1.4, with a 0.14-mm coverslip. Bars, 2 μm.
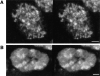
Figure 4
Fields of interphase nuclei in the amnioserosa of a fixed (A) and a living (B) embryo during stage 11 (elongated germ band) stained for Hrb57A. Both images are single confocal sections from stacks recorded as in Fig. 3. Width of the total field is 40 μm.
Figure 5
Transcripts from the 93D region and detection of the locus by oligonucleotide hybridization. (A) Schematic representation of the transcripts from the heat shock RNA-ω region in 93D. The gray box indicates the location of the intron which is spliced out of the smaller ω-c RNA. The repeat region of the long ω-n transcript is shown in black. The sequence of the fluorescently labeled oligonucleotide, Fl-P2, used as a probe for ω-n is given below a 29 base transcript sequence which is strongly conserved between the tandem repeats. B and C demonstrate that the probe specifically binds to 93D RNA in squashed polytene chromosomes. (B) DNA stained with DAPI. (C) 93D hybridized with FlP2 under nondenaturing conditions.
Figure 5
Transcripts from the 93D region and detection of the locus by oligonucleotide hybridization. (A) Schematic representation of the transcripts from the heat shock RNA-ω region in 93D. The gray box indicates the location of the intron which is spliced out of the smaller ω-c RNA. The repeat region of the long ω-n transcript is shown in black. The sequence of the fluorescently labeled oligonucleotide, Fl-P2, used as a probe for ω-n is given below a 29 base transcript sequence which is strongly conserved between the tandem repeats. B and C demonstrate that the probe specifically binds to 93D RNA in squashed polytene chromosomes. (B) DNA stained with DAPI. (C) 93D hybridized with FlP2 under nondenaturing conditions.
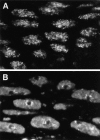
Figure 6
Association of Hrb57A with the 93D locus in situ. The RNA at the 93D locus in the nuclei of fixed embryos was detected by hybridization with the Fl-P2 oligonucleotide (left column) and the Hrb57A protein by mAb Q18 followed by a Cy3-coupled secondary antibody (center column). The superposition of both signals in an RGB image generates a yellow color where 93D and the protein are colocalized (right column). (A) A group of interphase nuclei from a nonstressed embryo. Overlay of 15 optical sections separated by 0.35 μm. (B) Higher magnification of a subregion from the field shown in A demonstrating the precise overlap of the 93D signals with some of the Hrb57A accumulations. (C) A group of interphase nuclei from an embryo that had been heat shocked at 37°C for 60 min before fixation. The restriction of Hrb57A to the 93D locus is evident.
Figure 8
Dynamic changes in the intranuclear distribution of Hrb57A during recovery from heat shock. Stereo images of amnioserosa nuclei reconstructed from five optical sections separated by 2 μm. Intranuclear accumulations of Hrb57A are numbered (see text). (A) 6 min after reducing the temperature to 25°C. (B) 10 min into recovery. Bar, 5 μm.
Similar articles
- Spatial organization of four hnRNP proteins in relation to sites of transcription, to nuclear speckles, and to each other in interphase nuclei and nuclear matrices of HeLa cells.
Mattern KA, van der Kraan I, Schul W, de Jong L, van Driel R. Mattern KA, et al. Exp Cell Res. 1999 Feb 1;246(2):461-70. doi: 10.1006/excr.1998.4267. Exp Cell Res. 1999. PMID: 9925762 - Separable roles in vivo for the two RNA binding domains of Drosophila A1-hnRNP homolog.
Zu K, Sikes ML, Beyer AL. Zu K, et al. RNA. 1998 Dec;4(12):1585-98. doi: 10.1017/s135583829898102x. RNA. 1998. PMID: 9848655 Free PMC article. - Nuclear RNA-protein interactions and messenger RNA processing.
Pederson T. Pederson T. J Cell Biol. 1983 Nov;97(5 Pt 1):1321-6. doi: 10.1083/jcb.97.5.1321. J Cell Biol. 1983. PMID: 6355116 Free PMC article. Review. - Beyond the heat shock pathway: Heat stress responses in Drosophila development.
Gibbs JR, Mei C, Wunderlich Z. Gibbs JR, et al. Dev Biol. 2025 Feb;518:53-60. doi: 10.1016/j.ydbio.2024.11.003. Epub 2024 Nov 16. Dev Biol. 2025. PMID: 39557149 Review.
Cited by
- Actin homolog MreB and RNA polymerase interact and are both required for chromosome segregation in Escherichia coli.
Kruse T, Blagoev B, Løbner-Olesen A, Wachi M, Sasaki K, Iwai N, Mann M, Gerdes K. Kruse T, et al. Genes Dev. 2006 Jan 1;20(1):113-24. doi: 10.1101/gad.366606. Genes Dev. 2006. PMID: 16391237 Free PMC article. - Visualization and tracking of single protein molecules in the cell nucleus.
Kues T, Peters R, Kubitscheck U. Kues T, et al. Biophys J. 2001 Jun;80(6):2954-67. doi: 10.1016/S0006-3495(01)76261-3. Biophys J. 2001. PMID: 11371468 Free PMC article. - The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms.
Makeyev AV, Liebhaber SA. Makeyev AV, et al. RNA. 2002 Mar;8(3):265-78. doi: 10.1017/s1355838202024627. RNA. 2002. PMID: 12003487 Free PMC article. Review. - Developmental regulation and complex organization of the promoter of the non-coding hsr(omega) gene of Drosophila melanogaster.
Lakhotia SC, Rajendra TK, Prasanth KV. Lakhotia SC, et al. J Biosci. 2001 Mar;26(1):25-38. doi: 10.1007/BF02708978. J Biosci. 2001. PMID: 11255511 - Characterization of the Drosophila BEAF-32A and BEAF-32B Insulator Proteins.
Avva SV, Hart CM. Avva SV, et al. PLoS One. 2016 Sep 13;11(9):e0162906. doi: 10.1371/journal.pone.0162906. eCollection 2016. PLoS One. 2016. PMID: 27622635 Free PMC article.
References
- Allis CD, Waring GL, Mahowald AP. Mass isolation of pole cells from Drosophila melanogaster. . Dev Biol. 1977;56:372–381. - PubMed
- Bendena WG, Ayme-Southgate A, Garbe JC, Pardue ML. Expression of heat-shock locus hsr-omega in nonstressed cells during development in Drosophila melanogaster. . Dev Biol. 1991;144:65–77. - PubMed
- Bonner JJ, Pardue ML. The effect of heat shock on RNA synthesis in Drosophilatissues. Cell. 1976;8:43–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases