Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody - PubMed (original) (raw)
Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody
T Miyata et al. J Neurosci. 1997.
Abstract
Cerebellar Purkinje cells are generated in the ventricular zone, migrate outward, and finally form a monolayer in the cortex. In reeler mice, however, most Purkinje cells cluster abnormally in subcortical areas. Reelin, the candidate reeler gene product recognized by the CR-50 monoclonal antibody, is concentrated in a cortical zone along which Purkinje cells are aligned linearly, implying that it may regulate their alignment. We used an in vitro system and a transplantation approach to analyze the function of Reelin. Explant culture for 7 d of cerebella isolated from wild-type and reeler mice at embryonic day 13 (E13) reproduced in a phenotype-dependent manner the two distinct arrangement patterns (linear vs clustered) of Purkinje cells. Extensive CR-50 binding to wild-type explants converted the linear pattern into a reeler-like, clustered pattern. On the other hand, when reeler explants lacking Reelin were crowned with an artificial layer of Reelin+ granule cells, some Reelin molecules were distributed into a superficial zone of the reeler explants, and Purkinje cells formed a linear pattern along the Reelin-rich overlay. This "rescue" effect was also inhibited by CR-50. Hence, Reelin is involved in the Purkinje cell alignment, and the lack of this activity may explain the malformation in reeler cerebella. We further injected Reelin+ granule cells into the fourth ventricle of E12-13 mice. Extensive incorporation of the injected Reelin+ cells into the ventricular zone, but not of Reelin- cells, forced Purkinje cells of the host cerebella to form an aberrant layer, suggesting that premigratory Purkinje cells may already be responsive to Reelin or Reelin-related signals.
Figures
Fig. 1.
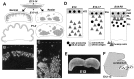
Experimental design to study the possible involvement of Reelin/CR-50 antigen in the arrangement of Purkinje cells. A, Schematic illustration showing behaviors of Purkinje cells (shaded) in vivo. In normal mice, Purkinje cells generated in a zone along the ventricular surface (V) migrate radially and then accumulate at superficial cortical areas near the pial surface (P) and form Purkinje cell layer (PCL; also see_D_). In reeler mice, however, Purkinje cells remain as clusters at deep cerebellar areas. B,C, Anti-calbindin immunostaining on sagittal cerebellar sections of normal (B) and reeler(C) mice at P2. Broken lines indicate the pial surface. Calbindin+ Purkinje cells are distributed distinctly between the two phenotypes. D, Spatiotemporal distributions of CR-50 antigen in the cerebellum of normal mice (Miyata et al., 1996). The molecule is produced by non-Purkinje neurons and presented extracellularly at the deeper half of the external granule layer (EGL), along which Purkinje cells are aligned, and not detected in reeler mice. E, Cerebellar explant isolated from E13 mice and mounted in collagen gel.F, Injection of granule cells into the fourth ventricle of E12–13 mouse embryos. Scale bar: B, C, 50 μm;E, 750 μm. NTZ, Nuclear transitory zone; PLZ, proliferative zone; PMZ, premigratory zone; ML, molecular layer;IGL, internal granular layer.
Fig. 4.
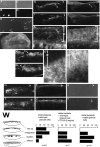
Reeler explants cocultured with granule cells. Cerebella of normal mice were dissociated at P4–8 (A, B), and granule cells were enriched (C, D), as described in Materials and Methods. Anti-calbindin staining (B, D; phase-contrast views of the same fields are shown in A and C) showed that Purkinje cells (two labeled cells in C) were excluded. By CR-50 staining (E), most of the enriched cells with round shape were positive (arrows); only one cell in this field (arrowhead) is CR-50−. These CR-50+ cells isolated from normal mice were also positive for zic, a marker of granule cells at this stage (Aruga et al., 1994) (not shown). F, With an overlay (ov) of such prepared normal-derived granule cells positive for Reelin/CR-50 antigen, cerebellar explants from reeler mice (ce) were crowned (a low-power phase-contrast view from the bottom of culture dish). G–L, An example of internal structures of reeler explants covered with the Reelin+ granule cells, examined by staining with anti-calbindin (G, I, K) and CR-50 (H, J, L) at 7 DIV. Stars indicate the overlay. Reelin+ cells were not found in explants, except for some areas in which the interface of the explants and overlays became ambiguous and the translocations of cells from each other were suggested (I, J). Purkinje cells were aligned along (line-up) or integrated (integration) into (some cells are found at the top of the overlay) the Reelin+ overlay. From the initial anti-calbindin staining of suspended cells for overlays, it was estimated that each overlay contained no more than 40 calbindin+ Purkinje cells. Moreover, by monitoring of overlays placed on fixed cerebella or collagen gel, only two to three Purkinje cells were detected throughout 10–15 planes chosen in steps of 100–120 μm. Therefore, most if not all calbindin+cells found in the overlays covering reeler explants were considered to have come from the underlying reeler_explants and are presented as integration. In cases in which cerebellar explants were prepared from reeler_embryos after pulse labeling with BrdU on E11 and E12 in utero, the majority of calbindin+ cells in the overlays were positive for BrdU (data not shown), also indicating that these cells may have migrated from the explants into the overlays.K and L are magnified views of the_arrowed portions in G and_H, respectively, and show that extracellular Reelin was condensed in the overlay but also distributed in the underlying space (arrowheads). M–P, The effect of CR-50 on a reeler explant covered with normal-derived granule cells. Arrowed portion in M is magnified in O. P is a CR-50-stained section adjacent to O and shows both intracellular labeling of overlying granule cells and extracellular binding of CR-50 during culture. Anti-calbindin staining (M–O) showed “clusters” of Purkinje cells or “diffuse” pattern as observed in nontreated reeler explants. The “line-up” pattern was not observed, but some Purkinje cells were “integrated” into the overlay (stars) as observed in the absence of CR-50.Q–V, A reeler explant covered with Reelin− granule cells. Neighboring sections at two separate planes across the long axis of the explant were stained with anti-calbindin (left), anti-zic (middle), and CR-50 (right). Granule cells purified from_reeler_ mice were not stained with CR-50, and overlays (stars) were identified by more intense labeling with anti-zic than explants containing endogenous zic+ cells that were scattered throughout. Purkinje cells were distributed randomly and often “clustered” (Q, T), as in untreated reeler explants. The results of all explants tested in these rescue experiments are presented in W. Scale bar: A–D, 75 μm; E, O, P, 30 μm, F, 1 mm; G–J, M, N, Q–V, 300 μm; K, L, 20 μm.
Fig. 2.
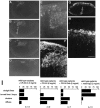
Untreated cerebellar explants derived from normal and reeler mice. Explants fixed at 7 DIV were sectioned across the long axis and stained with anti-calbindin. Sets of photomicrographs show distinct distribution patterns of Purkinje cells between a normal-derived explant (A–D) and a_reeler_-derived explant (E–H).D and H are magnified views of the indicated portion in C and F, respectively. I, Histograms showing the frequencies of occurrence of four “patterns” (vertical axis; also illustrated schematically on the left), which were extracted from photomicrographs of 10–15 separate sections covering the entire explants, in normal and reeler explants. For example, the wild-type case in A–D shows “straight lines” (A–D) and “turned lines” or “loops” (A, B), whereas the reeler case in_E–H_ shows “clusters” (F, H) and a “diffuse” pattern (E, G). One explant often had two or three patterns among the sections, and even one of the sections occasionally showed combined patterns. Therefore, the percentage for one of the patterns is calculated and presented independent of that for the remaining patterns, and the sum of the percentages exceeds 100%. J–Q are magnified views of superficial areas in explants (same magnification), and they show the spatial relationship between Purkinje cells and other layer-like structures identified in vitro. J–O, Sets of photomicrographs showing the relationships between calbindin+ Purkinje cells (J, M), extracellular Reelin/CR-50 antigen (asterisks in_K_ and N), and cells taking up BrdU (L, O) around the “straight line” (J–L) and “loop” (M–O), two PCL-like structures in wild-type explants. The wild-type explants were exposed to BrdU for 1 hr before fixation, and serial sections were stained with antibodies to these markers. In both structures, there was a lamination sequence from a layer or mass of cells taking up BrdU (L, O) to the “straight line” (J) or “loop” (M) formed by Purkinje cells, through an extracellularly CR-50 immunoreactive zone (K, N), which often overlapped the Purkinje cells. Broken lines in_J–L_ indicate the upper (pial) surface of explants. CR-50 immunoreactivity other than the bands (asterisk) in K and_N_ seems to correspond to granule neurons labeled intracellularly. P, Q, Double staining with anti-calbindin (P) and CR-50 (Q) on_reeler_ explants, in which Purkinje cells were clustered (P) and Reelin was not detected (Q). Scale bar: A–C, E–G, 200 μm;D, H, J–O, 50 μm.
Fig. 3.
Wild-type explants exposed to CR-50 and control IgG. Purkinje cells were identified with rat anti-type-I IP3R (A–E) or mouse anti-calbindin (F–H) monoclonal antibody at 7 DIV. In CR-50-exposed explants (A–F), Purkinje cells were found as clusters (the indicated site in B is magnified in E), which resembled those found in explants from reeler mice (also see Fig.2_H,P_). In the control explants (G, H), Purkinje cells showed “straight lines” as observed in nontreated wild-type explants (compare with Fig.2_A–D_). In F, a section adjacent to that shown in E was treated only with anti-calbindin, but tissue-bound CR-50 was visualized simultaneously with anti-mouse secondary antibody as fine puncta among the superficial zone above the clustered Purkinje cells. Scale bar: E, F, H, 50 μm; 200 μm in the remaining photographs. Graphs in_I_ show the frequencies of occurrence of the four “patterns” explained in Figure 2 and indicate that the blocking effect of CR-50 was reproduced by Fab treatment.
Fig. 5.
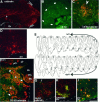
Effects of granule cell transplantation on embryonic Purkinje cells. Granule cells enriched as used for the overlays were labeled with DiI and injected into the fourth ventricle of wild-type mice at E13, as illustrated in Figure1_F_. Broken lines in A, C, D, F–I indicate the ventricular surface. Ch, Choroid plexus. A–D, Parasagittal sections of a wild-type host cerebellum fixed at P0. In sections that were double-stained with anti-spot35/calbindin (Yamakuni et al., 1984) (A, C) and CR-50 (B, C), most Purkinje cells were found in areas just below the external granular layer (EGL), but some Purkinje cells (arrowhead) were found ectopically in an area facing the fourth ventricle. In the same ventricular area, CR-50+cells formed a mass (thick arrows), although this area does not contain CR-50+ cells in untreated animals (Miyata et al., 1996). A view magnified further (C, double-exposed picture) shows that a group of Purkinje cells (the same_arrowhead_ as in A) is surrounded by the CR-50+ cells. To confirm the origin of these CR-50+ cells, DiI was visualized in the adjacent section (D). The pattern of DiI labeling matched the distribution of the CR-50+ cells along the ventricular surface. E–I, A wild-type host cerebellum that had been exposed to BrdU on E12 and fixed at P18. E is a set of traces of low-power photomicrographs of parasagittal sections stained with anti-spot35/calbindin, in which each dot represents one Purkinje cell. These sections were cut in steps of 120–150 μm and cover the entire cerebellum, and the traces were processed and arranged using Superpaint 3.5J (Aldus). Almost all Purkinje cells were found in the cortex, but ectopic Purkinje cells (arrows) were also found in an area near the ventricular surface between the cerebellar peduncle (stars) and the site where choroid plexus begins, spreading over nearly 1 mm along the lateral-to-medial axis (from section 4 to 10). The corresponding but grafted cell-free area in the opposite side (sections_14–19_) was empty of Purkinje cells. F, Photomicrograph of section 7, double-exposed for CR-50 (green) and anti-spot35 (red). In addition to Purkinje cells localized in the normal cortical positions [calbindin+ cells comprising the Purkinje cell layer (PCL) and molecular layer (ML)], another population of Purkinje cells was found near the ventricular surface. The latter ectopic Purkinje cells were surrounded by CR-50+cells and showed a layer-like pattern with their cell bodies (arrows) and dendrites (asterisks), which were sorted out from each other. Endogenous CR-50 immunoreactivity was already lost in the granular layer (GL) of the host.G, Double staining with anti-BrdU (green) and anti-calbindin (red). The arrowed nucleus is BrdU+, indicating that the ectopic Purkinje cell was generated in the host cerebellum on E12 (labeling indices were ∼50% in the ectopic Purkinje cells and ∼35% in PCL). H, Double staining with anti-calbindin (green) and anti-zic (red).I, DiI (red) was visualized in an anti-zic (green)-stained section. Scale bar:A, 200 μm; B, F, 100 μm; C, D, G–I, 50 μm.
Similar articles
- Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of reelin.
Goldowitz D, Cushing RC, Laywell E, D'Arcangelo G, Sheldon M, Sweet HO, Davisson M, Steindler D, Curran T. Goldowitz D, et al. J Neurosci. 1997 Nov 15;17(22):8767-77. doi: 10.1523/JNEUROSCI.17-22-08767.1997. J Neurosci. 1997. PMID: 9348346 Free PMC article. - Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum.
Miyata T, Ono Y, Okamoto M, Masaoka M, Sakakibara A, Kawaguchi A, Hashimoto M, Ogawa M. Miyata T, et al. Neural Dev. 2010 Sep 1;5:23. doi: 10.1186/1749-8104-5-23. Neural Dev. 2010. PMID: 20809939 Free PMC article. - Trajectory Analysis Unveils Reelin's Role in the Directed Migration of Granule Cells in the Dentate Gyrus.
Wang S, Brunne B, Zhao S, Chai X, Li J, Lau J, Failla AV, Zobiak B, Sibbe M, Westbrook GL, Lutz D, Frotscher M. Wang S, et al. J Neurosci. 2018 Jan 3;38(1):137-148. doi: 10.1523/JNEUROSCI.0988-17.2017. Epub 2017 Nov 14. J Neurosci. 2018. PMID: 29138282 Free PMC article. - [Cytoarchitectonic abnormality in the facial nucleus of the reeler mouse].
Terashima T, Setsu T, Kikkawa S, Ikeda Y. Terashima T, et al. Kaibogaku Zasshi. 1999 Aug;74(4):411-20. Kaibogaku Zasshi. 1999. PMID: 10496086 Review. Japanese. - Role of reelin in the control of brain development.
Curran T, D'Arcangelo G. Curran T, et al. Brain Res Brain Res Rev. 1998 May;26(2-3):285-94. doi: 10.1016/s0165-0173(97)00035-0. Brain Res Brain Res Rev. 1998. PMID: 9651544 Review.
Cited by
- Fyn Tyrosine Kinase as Harmonizing Factor in Neuronal Functions and Dysfunctions.
Matrone C, Petrillo F, Nasso R, Ferretti G. Matrone C, et al. Int J Mol Sci. 2020 Jun 22;21(12):4444. doi: 10.3390/ijms21124444. Int J Mol Sci. 2020. PMID: 32580508 Free PMC article. Review. - Mutation in the gene encoding lysosomal acid phosphatase (Acp2) causes cerebellum and skin malformation in mouse.
Mannan AU, Roussa E, Kraus C, Rickmann M, Maenner J, Nayernia K, Krieglstein K, Reis A, Engel W. Mannan AU, et al. Neurogenetics. 2004 Dec;5(4):229-38. doi: 10.1007/s10048-004-0197-9. Epub 2004 Oct 22. Neurogenetics. 2004. PMID: 15503243 - Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro.
Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, Weeber EJ, Manzoni OJ, Chavis P. Sinagra M, et al. J Neurosci. 2005 Jun 29;25(26):6127-36. doi: 10.1523/JNEUROSCI.1757-05.2005. J Neurosci. 2005. PMID: 15987942 Free PMC article. - An expandable embryonic stem cell-derived Purkinje neuron progenitor population that exhibits in vivo maturation in the adult mouse cerebellum.
Higuera GA, Iaffaldano G, Bedar M, Shpak G, Broersen R, Munshi ST, Dupont C, Gribnau J, de Vrij FMS, Kushner SA, De Zeeuw CI. Higuera GA, et al. Sci Rep. 2017 Aug 18;7(1):8863. doi: 10.1038/s41598-017-09348-1. Sci Rep. 2017. PMID: 28821816 Free PMC article. - Pattern formation during development of the embryonic cerebellum.
Dastjerdi FV, Consalez GG, Hawkes R. Dastjerdi FV, et al. Front Neuroanat. 2012 Apr 4;6:10. doi: 10.3389/fnana.2012.00010. eCollection 2012. Front Neuroanat. 2012. PMID: 22493569 Free PMC article.
References
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972;145:353–398. - PubMed
- Altman J, Bayer SA. Embryonic development of the rat cerebellum. I. Delineation of the cerebellar primordium and early cell movements. J Comp Neurol. 1985;231:1–26. - PubMed
- Aruga J, Yokota N, Hashimoto M, Furuichi T, Fukuda M, Mikoshiba K. A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J Neurochem. 1994;63:1880–1890. - PubMed
- Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–260. - PubMed
- Bravin M, Rossi F, Strata P. Different climbing fibres innervate separate dendritic regions of the same Purkinje cell in hypogranular cerebellum. J Comp Neurol. 1995;357:395–407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources