The role of an amygdalo-nigrostriatal pathway in associative learning - PubMed (original) (raw)
The role of an amygdalo-nigrostriatal pathway in associative learning
J S Han et al. J Neurosci. 1997.
Abstract
The present study examined the role of an amygdalo-nigrostriatal pathway in associative learning. An asymmetrical lesion model was used to test whether a circuit from the amygdala central nucleus to the dorsolateral striatum, via the substantia nigra, is critical for mediating conditioned orienting responses. Rats with an asymmetrical lesion, consisting of neurotoxic removal of central nucleus neurons in one hemisphere and depletion of the dopamine innervation of the dorsolateral striatum in the contralateral hemisphere, failed to acquire conditioned orienting responses. In contrast, the asymmetrical lesion had no effect on spontaneous orienting or learning another response directed to the source of the food unconditioned stimulus in the same task. A second experiment tested the effect of reversible inactivation of the dorsolateral striatum contralateral to a neurotoxic central nucleus lesion on acquisition of the conditioned orienting response. Although inactivation did not affect spontaneous orienting, rats failed to acquire the conditioned orienting response during sessions in which inactivation occurred. Immediately after the inactivation procedure was terminated, however, a significant increase in orienting to the conditioned stimulus was evident. These data support the interpretation that the dorsolateral striatum provides a route for the expression of the conditioned orienting response but is not essential for acquisition of this learned behavior.
Figures
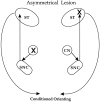
Fig. 1.
The combination of a unilateral lesion of CN in one hemisphere (indicated by the X on the top right in the schematic) with a contralateral lesion of the dorsolateral striatum (X on the lower left) prevents output dependent on the amygdalo-nigrostriatal circuitry shown in the schematic.
Fig. 2.
a, Diagram of the amygdala complex with the position of the photomicrograph (shown in b) demarcated by the enclosed box and the area of the lesion identified by broken lines. b, Heavy gliosis marks the site of the lesion in the CN surrounding the stria terminalis. c, Autoradiogram shows DA uptake sites labeled with [3H]Mazindol. High levels of binding (dark area) are evident throughout the intact striatum on the right. The site of 6-OHDA administration on the_left_ exhibits low binding, indicative of a loss of DA innervation. Average lesion area was 3.03 mm2, and radioactive density averaged 1.88 nCi/mg relative to 8.35 nCi/mg on the contralateral side. ABL, Basolateral nucleus;CN, central nucleus; ME, medial nucleus;st, stria terminalis.
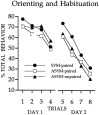
Fig. 3.
Responses to the visual cue during two sessions of light-alone presentations. All groups showed comparable orienting and habituation during these trials.
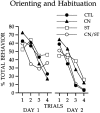
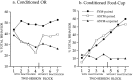
Fig. 4.
Conditioned orienting behavior (a) and conditioned food-cup behavior (b) during 10 sessions in which the light CS was paired with the food US. Rats with the asymmetrical lesion (group CN/ST) failed to acquire conditioned orienting behavior relative to the acquisition displayed by all other comparison groups. All groups showed comparable acquisition of the conditioned food-cup response.
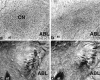
Fig. 5.
Photomicrographs show Nissl-stained sections (top) and gold chloride myelin-stained sections (bottom) taken in the coronal plane through the amygdala central nucleus. a and c are from a control brain. The neurotoxic lesion brain in b shows heavy gliosis in the CN. Spared neurons are evident in the far lateral CN. However, d shows intact myelin in the lesion area (shown in b). ABL, Basolateral nucleus;CN, central nucleus; st, stria terminalis.
Fig. 6.
Rats in all groups showed initial orienting and comparable habituation to the visual CS.
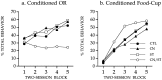
Fig. 7.
a, Graph shows the orienting response during conditioning sessions. During the inactivation sessions (1–4), the ASYM-paired did not differ from the ASYM-unpaired, where as the SYM-paired exhibited significantly greater conditioned ORs than each of the other groups. In addition, in the first session without inactivation (session 5), the ASYM-paired exhibited greater conditioned ORs than in the previous inactivation session (session 4).b, Rats in the conditioning groups that received paired light/food trials developed conditioned food-cup responses that were unaffected by STR inactivation. Compare SYM-paired and ASYM-paired groups with the ASYM-unpaired group.
Similar articles
- Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation.
El-Amamy H, Holland PC. El-Amamy H, et al. Eur J Neurosci. 2007 Mar;25(5):1557-67. doi: 10.1111/j.1460-9568.2007.05402.x. Eur J Neurosci. 2007. PMID: 17425582 Free PMC article. - Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting.
McDannald M, Kerfoot E, Gallagher M, Holland PC. McDannald M, et al. Eur J Neurosci. 2004 Jul;20(1):240-8. doi: 10.1111/j.0953-816X.2004.03458.x. Eur J Neurosci. 2004. PMID: 15245496 - Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food.
Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Lee HJ, et al. J Neurosci. 2005 Apr 13;25(15):3881-8. doi: 10.1523/JNEUROSCI.0416-05.2005. J Neurosci. 2005. PMID: 15829640 Free PMC article. - Amygdalo-striatal interaction in the enhancement of stimulus salience in associative learning.
Esber GR, Torres-Tristani K, Holland PC. Esber GR, et al. Behav Neurosci. 2015 Apr;129(2):87-95. doi: 10.1037/bne0000041. Epub 2015 Mar 2. Behav Neurosci. 2015. PMID: 25730120 Free PMC article. - Ibotenic acid lesions in the pedunculopontine region result in recovery of visual orienting in the hemianopic cat.
Durmer JS, Rosenquist AC. Durmer JS, et al. Neuroscience. 2001;106(4):765-81. doi: 10.1016/s0306-4522(01)00321-9. Neuroscience. 2001. PMID: 11682162
Cited by
- Optogenetic inhibition of the caudal substantia nigra inflates behavioral responding to uncertain threat and safety.
Wright KM, Cieslewski S, Chu A, McDannald MA. Wright KM, et al. Behav Neurosci. 2023 Dec;137(6):347-355. doi: 10.1037/bne0000568. Epub 2023 Oct 5. Behav Neurosci. 2023. PMID: 37796586 - Anxiety in synucleinopathies: neuronal circuitry, underlying pathomechanisms and current therapeutic strategies.
Lai TT, Gericke B, Feja M, Conoscenti M, Zelikowsky M, Richter F. Lai TT, et al. NPJ Parkinsons Dis. 2023 Jun 22;9(1):97. doi: 10.1038/s41531-023-00547-4. NPJ Parkinsons Dis. 2023. PMID: 37349373 Free PMC article. Review. - Optogenetic inhibition of the caudal substantia nigra inflates behavioral responding to uncertain threat and safety.
Wright KM, Cieslewski S, Chu A, McDannald MA. Wright KM, et al. bioRxiv [Preprint]. 2023 Feb 18:2023.02.18.529041. doi: 10.1101/2023.02.18.529041. bioRxiv. 2023. PMID: 36824795 Free PMC article. Updated. Preprint. - Abstinence-Induced Nicotine Seeking Relays on a Persistent Hypoglutamatergic State within the Amygdalo-Striatal Neurocircuitry.
Domi A, Domi E, Lagstrom O, Gobbo F, Jerlhag E, Adermark L. Domi A, et al. eNeuro. 2023 Feb 21;10(2):ENEURO.0468-22.2023. doi: 10.1523/ENEURO.0468-22.2023. Print 2023 Feb. eNeuro. 2023. PMID: 36754627 Free PMC article. - The role of the bed nucleus of the stria terminalis in the motivational control of instrumental action.
Ge M, Balleine BW. Ge M, et al. Front Behav Neurosci. 2022 Nov 21;16:968593. doi: 10.3389/fnbeh.2022.968593. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36478779 Free PMC article.
References
- Burwell RD, Gallagher M. A longitudinal study of reaction time performance in Long–Evans rats. Neurobiol Aging. 1993;14:57–64. - PubMed
- Carli M, Evenden JL, Robbins TW. Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature. 1985;313:679–682. - PubMed
- Carli M, Jones GH, Robbins TW. Effects of unilateral dorsal and ventral striatal dopamine depletion on visual neglect in the rat: a neural and behavioral analysis. Neuroscience. 1989;29:309–327. - PubMed
- Fairley PC, Marshall JF. Dopamine in the lateral caudate-putamen of the rat is essential for somatosensory orientation. Behav Neurosci. 1986;100:652–663. - PubMed
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1990;180:545–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources