Channel formation by antiapoptotic protein Bcl-2 - PubMed (original) (raw)
Channel formation by antiapoptotic protein Bcl-2
S L Schendel et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Bcl-2 is the prototypical member of a large family of apoptosis-regulating proteins, consisting of blockers and promoters of cell death. The three-dimensional structure of a Bcl-2 homologue, Bcl-XL, suggests striking similarity to the pore-forming domains of diphtheria toxin and the bacterial colicins, prompting exploration of whether Bcl-2 is capable of forming pores in lipid membranes. Using chloride efflux from KCl-loaded unilamellar lipid vesicles as an assay, purified recombinant Bcl-2 protein exhibited pore-forming activity with properties similar to those of the bacterial toxins, diphtheria toxin, and colicins, i.e., dependence on low pH and acidic lipid membranes. In contrast, a mutant of Bcl-2 lacking the two core hydrophobic alpha-helices (helices 5 and 6), predicted to be required for membrane insertion and channel formation, produced only nonspecific effects. In planar lipid bilayers, where detection of single channels is possible, Bcl-2 formed discrete ion-conducting, cation-selective channels, whereas the Bcl-2 (Deltah5, 6) mutant did not. The most frequent conductance observed (18 +/- 2 pS in 0.5 M KCl at pH 7.4) is consistent with a four-helix bundle structure arising from Bcl-2 dimers. However, larger channel conductances (41 +/- 2 pS and 90 +/- 10 pS) also were detected with progressively lower occurrence, implying the step-wise formation of larger oligomers of Bcl-2 in membranes. These findings thus provide biophysical evidence that Bcl-2 forms channels in lipid membranes, suggesting a novel function for this antiapoptotic protein.
Figures
Figure 1
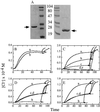
Bcl-2 forms pH-dependent pores in acidic-lipid containing liposomes. (A) The His6-Bcl-2 (ΔTM) protein (left) and mutant His6-Bcl-2 (Δh5, 6) (ΔTM) protein (right) were purified using Ni-chelation affinity chromatography. Coomassie-stained SDS/PAGE gels of the His6-Bcl-2 (ΔTM) and His6-Bcl-2 (Δh5, 6) (ΔTM) proteins are shown, demonstrating >95% purity of the intact recombinant proteins (molecular mass markers are shown in kilodaltons). (B) Bcl-2 and colicin E1 proteins are contrasted with regards to Cl− efflux from KCl-loaded 70% DOPC/30% DOPG liposomes at pH 4.0. His6-Bcl-2 (ΔTM) (b) or colicin E1 (a) were added at _t_0 in dimethyl glutaric acid buffer at ≈350 ng/ml and 50 ng/ml, respectively. Arrows indicate addition of 0.1% Triton X-100 to release residual Cl− from the vesicles. (C) Bcl-2-mediated (300 ng/ml) Cl− efflux was compared at various pHs, including pH 4.0 (a), 4.5 (b), 5.0 (c), 5.5 (d), and 6.0 (e). (D) Wild-type (a, b) and mutant (c, d) Bcl-2 proteins were evaluated for ability to induce Cl− efflux from acidic 70% DOPC/30% DOPG (a, c) and neutral 100% DOPC (b, d) vesicles. Arrows indicate addition of 0.1% Triton X-100 to induce release of residual Cl−. (E) Wild-type His6-Bcl-2 (ΔTM) (a, b) and mutant His6-Bcl-2 (Δh5, 6) (ΔTM) (c, d) proteins (300 ng/ml) were compared in 70% DOPC/30% DOPG liposomes. Cl− efflux was measured at pH 4.0 (a, c) and 5.5 (b, d).
Figure 2
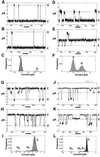
Bcl-2 forms discrete channels in planar lipid bilayers. Single-channel recordings were obtained from preformed lipid bilayers in symmetric 0.5 M KCl supplemented with 1 mM CaCl2 and 10 mM Hepes, pH 7.4 after addition of 50 μg/ml His6-Bcl-2 (ΔTM) protein. The currents of the closed (C) and open (O) states are indicated by the solid lines. The dotted lines define the range set to discriminate the transitions between states and were based on signal to noise ratio measurements (33). For the records obtained at + 100 mV (A_–_F), an upward deflection indicates channel opening, whereas a downward deflection represents channel opening at −100 mV (G_–_L). Current histograms and Gaussian fits (C, F, I, L) reflect results obtained in the range −100 mV ≤ V ≤ 100 mV from continuous segments of a record lasting several minutes. (A_–_C) Records chosen to demonstrate the 41 ± 2 pS channel, where A and B are representative current records and C is the current histogram with Gaussian fit of the data, showing relative occurrence of the open (O) and closed (C) channel states. The record was derived from a burst of 40 pS channel activity occurring in the absence of 20 pS channels. The probability of the channel being closed (_P_c) ≥ 0.9, under these experimental conditions. The open channel lifetimes (τo) for the γ= 41 pS conductance channel were well fitted by a sum of two exponentials, τo1 = 6.4 ± 1 msec, τo2 = 11.2 ± 3 msec and the closed times by, τc1 = 0.6 ± 0.2, τc2 = 17.7 ± 3.0 msec (n = 3). D_–_F demonstrate the 18 ± 2 pS channel, where D shows a record where the channel is mostly closed (five openings over the time course presented), E an example where channel is mostly open over the time shown (note transient openings of a second ≈20 pS channel after ≈250 msec), and F represents the current histogram. The data are derived from a burst of purely ≈20 pS channel activity. The region of the record selected for histogram preparation was biased toward a period of high-frequency channel opening and is intended to show the high signal-to-noise ratio even for this small ≈20 pS channel. (G_–_I) Examples of the ≈40 pS (marked by solid line and flanking dashed lines) and ≈20 pS (not marked) channels recorded at −100 mV (downward deflections), demonstrating similar character to the recordings at +100 mV. The mixture of ≈20 pS and ≈40 pS channel activities shown in this record is more typical of Bcl-2 channel formation than the monoconductance channel activities that were presented in A_–_F for purposes of illustration. The current histogram (I) notes the frequency of occurrence of the open states for the ≈20 pS (O1) and ≈40 pS (O2) channels, and is representative of the relative times the ≈20 pS and the ≈40 pS channels occupy the closed and opened states. (J_–_L) The less common 90 ± 10 pS channel. Note interspersed ≈20 pS and ≈40 pS conductances. (The ≈40 pS channel is denoted by the solid line for comparison.) The current histogram (L) demonstrates the relative frequency of occurrence of the open states for the ≈20 pS (O1), ≈40 pS (O2), and ≈90 pS (O3) channels. The ≈90 pS channel occurrence is too infrequent to be visually discerned in the plot. The particular records presented (J, K) are biased to show examples of this larger conductance channel activity.
Figure 3
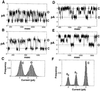
Bcl-2 channels in lipid bilayers at pH 5.4. Single-channel recordings are shown from lipid bilayers in symmetric 0.5 M KCl, 1 mM CaCl2 adjusted to pH 5.4 after addition of 50 μg/ml His6-Bcl-2 (ΔTM) protein. (A_–_C) Sample records obtained at V = 100 mV, demonstrating the occurrence of the 26 pS channels. _D_-F correspond to records at V = −100 mV, showing channels of 10 pS and 20 pS. Other conditions are as for Fig. 2.
Figure 4
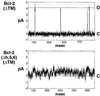
The Bcl-2 (Δh5, 6) mutant fails to form channels. Membrane currents from lipid bilayers containing His6-Bcl-2 (ΔTM) (A) and the deletion mutant lacking helices 5 and 6 (B). The final protein concentrations were 50 μg/ml. Currents recorded at 100 mV in symmetric 0.5 M KCl, 1 mM CaCl2, 5 mM Hepes, pH 7.4. Note the occurrence of stray, erratic fluctuations in membrane current produced by the deletion mutant contrasted with the square events produced by the wild-type Bcl-2. Data representative of seven experiments.
Similar articles
- Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2.
Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Schlesinger PH, et al. Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11357-62. doi: 10.1073/pnas.94.21.11357. Proc Natl Acad Sci U S A. 1997. PMID: 9326614 Free PMC article. - Ion channel activity of the BH3 only Bcl-2 family member, BID.
Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL, Reed JC. Schendel SL, et al. J Biol Chem. 1999 Jul 30;274(31):21932-6. doi: 10.1074/jbc.274.31.21932. J Biol Chem. 1999. PMID: 10419515 - Bcl-x(L) forms an ion channel in synthetic lipid membranes.
Minn AJ, Vélez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB. Minn AJ, et al. Nature. 1997 Jan 23;385(6614):353-7. doi: 10.1038/385353a0. Nature. 1997. PMID: 9002522 - Bcl-2 family proteins as ion-channels.
Schendel SL, Montal M, Reed JC. Schendel SL, et al. Cell Death Differ. 1998 May;5(5):372-80. doi: 10.1038/sj.cdd.4400365. Cell Death Differ. 1998. PMID: 10200486 Review. - Emerging issues of connexin channels: biophysics fills the gap.
Harris AL. Harris AL. Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
- Acidic pH promotes oligomerization and membrane insertion of the BclXL apoptotic repressor.
Bhat V, Kurouski D, Olenick MB, McDonald CB, Mikles DC, Deegan BJ, Seldeen KL, Lednev IK, Farooq A. Bhat V, et al. Arch Biochem Biophys. 2012 Dec 1;528(1):32-44. doi: 10.1016/j.abb.2012.08.009. Epub 2012 Aug 30. Arch Biochem Biophys. 2012. PMID: 22960132 Free PMC article. - Evidence for crucial electrostatic interactions between Bcl-2 homology domains BH3 and BH4 in the anti-apoptotic Nr-13 protein.
Lalle P, Aouacheria A, Dumont-Miscopein A, Jambon M, Venet S, Bobichon H, Colas P, Deléage G, Geourjon C, Gillet G. Lalle P, et al. Biochem J. 2002 Nov 15;368(Pt 1):213-21. doi: 10.1042/BJ20020836. Biochem J. 2002. PMID: 12133006 Free PMC article. - A multimeric model for murine anti-apoptotic protein Bcl-2 and structural insights for its regulation by post-translational modification.
Mathura VS, Soman KV, Varma TK, Braun W. Mathura VS, et al. J Mol Model. 2003 Oct;9(5):298-303. doi: 10.1007/s00894-003-0152-y. Epub 2003 Aug 30. J Mol Model. 2003. PMID: 14517609 - Blocking cytochrome c activity within intact neurons inhibits apoptosis.
Neame SJ, Rubin LL, Philpott KL. Neame SJ, et al. J Cell Biol. 1998 Sep 21;142(6):1583-93. doi: 10.1083/jcb.142.6.1583. J Cell Biol. 1998. PMID: 9744886 Free PMC article. - The Bax BH3 peptide H2-H3 promotes apoptosis by inhibiting Bcl-2's pore-forming and anti-Bax activities in the membrane.
Peng J, Lapolla SM, Zhang Z, Lin J. Peng J, et al. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009 Aug;26(4):829-35. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009. PMID: 19813621 Free PMC article.
References
- Yang E, Korsmeyer S J. Blood. 1996;88:386–401. - PubMed
- Hanada M, Aimé-Sempé C, Sato T, Reed J C. J Biol Chem. 1995;270:11962–11968. - PubMed
- Yin X M, Oltvai Z N, Korsmeyer S J. Nature (London) 1994;369:321–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials