A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity - PubMed (original) (raw)
A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity
S Yamamoto et al. Proc Natl Acad Sci U S A. 1997.
Free PMC article
Abstract
We have characterized a nontoxic mutant of cholera toxin (CT) as a mucosal adjuvant in mice. The mutant CT was made by substitution of serine with phenylalanine at position 61 of the A subunit (S61F), which resulted in loss of ADP ribosyltransferase activity and toxicity. Mice were intranasally immunized with ovalbumin, tetanus toxoid, or influenza virus either alone or together with mutant CT S61F, native CT, or recombinant CT-B. Mice immunized with these proteins plus S61F showed high serum titers of protein-specific IgG and IgA antibodies that were comparable to those induced by native CT. Further, high protein-specific IgA antibody responses were observed in nasal and vaginal washes, saliva, and fecal extracts as well as increased numbers of IgG and IgA antibody forming cells in cervical lymph nodes and lung tissues of mice intranasally immunized with these proteins and S61F or native CT, but not with recombinant CT-B or protein alone. Both S61F and native CT enhanced the induction of ovalbumin-specific CD4(+) T cells in lung and splenic tissues, and these T cells produced a Th2-type cytokine pattern of interleukin 4 (IL-4), IL-5, IL-6, and IL-10 as determined by analysis of secreted proteins and by quantitation of cytokine-specific mRNA. These results have shown that mutant CT S61F is an effective mucosal adjuvant when administrated intranasally and induces mucosal and systemic antibody responses which are mediated by CD4(+) Th2-type cells.
Figures
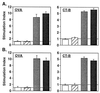
Figure 1
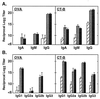
Serum OVA- and CT-B-specific IgA, IgM, and IgG (A) and IgG subclass (B) responses on day 21 following intranasal immunization with OVA combined with mCT S61F or nCT as adjuvants were determined by endpoint ELISA. Groups of C57BL/6 mice were immunized with 100 μg of OVA alone (□) or together with 5 μg of rCT-B (▨), 0.5 μg of nCT (░⃞), or 5 μg of mCT S61F (▪) on days 0, 7, and 14. Serum samples were collected 1 week after the last immunization. Bars represent the mean Ab titer ± 1 SE in each group. Each group consisted of five mice, and the data are representative of three separate experiments.
Figure 2
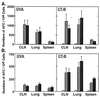
Numbers of OVA- and CT-B-specific IgG (A) and IgA (B) AFC in CLN, lung tissues and spleen following intranasal immunization with OVA combined with mCT S61F or nCT as mucosal adjuvants were determined by ELISPOT assay. Groups of C57BL/6 mice were immunized with 100 μg of OVA alone (□) or together with 5 μg of rCT-B (▨), 0.5 μg of nCT (░⃞), or 5 μg of mCT S61F (▪) on days 0, 7, and 14. Samples were collected 1 week after the last immunization. Bars represent the mean numbers of AFC ± 1 SE and each group contained five mice. The data are representative of three separate experiments.
Figure 3
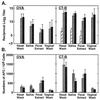
OVA- and CT-B-specific IgA Ab responses in mucosal secretions were determined by ELISA (A) and numbers of IgA AFC in mucosal tissues by ELISPOT assay (B) following intranasal immunization with OVA combined with mCT S61F or nCT as mucosal adjuvants. Groups of C57BL/6 mice were immunized with 100 μg of OVA alone (□) or together with 5 μg of rCT-B (▨), 0.5 μg of nCT (░⃞), or 5 μg of mCT S61F (▪) on days 0, 7, and 14. Tissue samples and external secretions were taken 1 week after the last immunization. Bars represent the mean Ab titer or numbers of AFC ± 1 SE in each group. Each group contained five mice and the data are representative of three separate experiments.
Figure 4
OVA- and CT-B-specific CD4+ T cell proliferative responses isolated from lung (A) and spleen (B). Groups of C57BL/6 mice were immunized with 100 μg of OVA alone (□) or together with 10 μg of rCT-B (▨), 0.5 μg of nCT (░⃞), or 5 μg of mCT S61F (▪) on days 0, 7, and 14. Bars represent the mean stimulation index ± 1 SE and each group contained five mice. The data were similar and are representative of four separate experiments.
Figure 5
Cytokine production from OVA-specific CD4+ T cells isolated from lung tissues. Molecules of cytokine-specific mRNA were determined by quantitative RT-PCR. Cytokine protein production was determined by ELISA. The scale of each figure corresponds to mRNA molecules and protein levels produced by nonimmunized CD4+ T cells stimulated with anti-CD3 mAb. ND, not detected; IFN-γ, interferon γ. Bars represent the mean cytokine profile ± 1 SE in each group. The data are representative of four separate experiments.
Similar articles
- Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues.
Xu-Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, Burrows PD, Elson CO, Pillai S, McGhee JR. Xu-Amano J, et al. J Exp Med. 1993 Oct 1;178(4):1309-20. doi: 10.1084/jem.178.4.1309. J Exp Med. 1993. PMID: 8376936 Free PMC article. - Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue.
Hagiwara Y, McGhee JR, Fujihashi K, Kobayashi R, Yoshino N, Kataoka K, Etani Y, Kweon MN, Tamura S, Kurata T, Takeda Y, Kiyono H, Fujihashi K. Hagiwara Y, et al. J Immunol. 2003 Feb 15;170(4):1754-62. doi: 10.4049/jimmunol.170.4.1754. J Immunol. 2003. PMID: 12574339 - Impaired mucosal immune responses in interleukin 4-targeted mice.
Vajdy M, Kosco-Vilbois MH, Kopf M, Köhler G, Lycke N. Vajdy M, et al. J Exp Med. 1995 Jan 1;181(1):41-53. doi: 10.1084/jem.181.1.41. J Exp Med. 1995. PMID: 7807021 - Mucosal immunity: regulation by helper T cells and a novel method for detection.
Jackson RJ, Marinaro M, VanCott JL, Yamamoto M, Okahashi N, Fujihashi K, Kiyono H, Chatfield SN, McGhee JR. Jackson RJ, et al. J Biotechnol. 1996 Jan 26;44(1-3):209-16. doi: 10.1016/0168-1656(95)00095-X. J Biotechnol. 1996. PMID: 8717406 Review. - The common mucosal immune system: from basic principles to enteric vaccines with relevance for the female reproductive tract.
McGhee JR, Xu-Amano J, Miller CJ, Jackson RJ, Fujihashi K, Staats HF, Kiyono H. McGhee JR, et al. Reprod Fertil Dev. 1994;6(3):369-79. doi: 10.1071/rd9940369. Reprod Fertil Dev. 1994. PMID: 7831485 Review.
Cited by
- Mucosal immunogenicity and adjuvant activity of the recombinant A subunit of the Escherichia coli heat-labile enterotoxin.
De Haan L, Holtrop M, Verweij WR, Agsteribbe E, Wilschut J. De Haan L, et al. Immunology. 1999 Aug;97(4):706-13. doi: 10.1046/j.1365-2567.1999.00817.x. Immunology. 1999. PMID: 10457227 Free PMC article. - Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant.
Richards CM, Aman AT, Hirst TR, Hill TJ, Williams NA. Richards CM, et al. J Virol. 2001 Feb;75(4):1664-71. doi: 10.1128/JVI.75.4.1664-1671.2001. J Virol. 2001. PMID: 11160664 Free PMC article. - Nasal delivery of antigen with the B subunit of Escherichia coli heat-labile enterotoxin augments antigen-specific T-cell clonal expansion and differentiation.
Apostolaki M, Williams NA. Apostolaki M, et al. Infect Immun. 2004 Jul;72(7):4072-80. doi: 10.1128/IAI.72.7.4072-4080.2004. Infect Immun. 2004. PMID: 15213152 Free PMC article. - Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens.
Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, Kok WL, Cole S, Ho LP, Lambe T, Puthia M, Svanborg C, Scherer EM, Krashias G, Williams A, Blattman JN, Greenberg PD, Flavell RA, Moghaddam AE, Sheppard NC, Sattentau QJ. Wegmann F, et al. Nat Biotechnol. 2012 Sep;30(9):883-8. doi: 10.1038/nbt.2344. Nat Biotechnol. 2012. PMID: 22922673 Free PMC article. - Mucosal immunity and tolerance: relevance to vaccine development.
Czerkinsky C, Anjuere F, McGhee JR, George-Chandy A, Holmgren J, Kieny MP, Fujiyashi K, Mestecky JF, Pierrefite-Carle V, Rask C, Sun JB. Czerkinsky C, et al. Immunol Rev. 1999 Aug;170(1):197-222. doi: 10.1111/j.1600-065x.1999.tb01339.x. Immunol Rev. 1999. PMID: 10566152 Free PMC article. Review.
References
- Elson C O, Ealding W. J Immunol. 1984;132:2736–2741. - PubMed
- Elson C O, Ealding W. J Immunol. 1984;133:2892–2897. - PubMed
- Clements J D, Hartzog N M, Lyon F L. Vaccine. 1988;6:269–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DE 04217/DE/NIDCR NIH HHS/United States
- DK 44240/DK/NIDDK NIH HHS/United States
- R29 DE012242/DE/NIDCR NIH HHS/United States
- AI 18958/AI/NIAID NIH HHS/United States
- N01 AI065299/AI/NIAID NIH HHS/United States
- N01 AI065298/AI/NIAID NIH HHS/United States
- R01 AI018958/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous