Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy - PubMed (original) (raw)
Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy
W Yu et al. J Cell Biol. 1997.
Abstract
Considerable evidence links urokinase plasminogen activator (uPA) bound to its surface receptor (uPAR) with enhanced invasiveness of cancer cells. By blocking uPAR expression in human epidermoid carcinoma cells (HEp3), we have now identified an additional and novel in vivo function for this receptor by showing that receptor-deficient cells enter a state of dormancy reminiscent of that observed in human cancer metastasis. Its main characteristic is survival without signs of progressive growth. Five clones transfected with a vector expressing uPAR antisense RNA under the beta-actin promoter were isolated and shown to have uPAR (at the mRNA and protein levels) reduced by 50 to 80%; four clones, transfected with vector alone and having uPAR levels similar to those of parental cells, served as controls. In confirmation of our previous results, reduced uPAR always coincided with a significantly reduced invasiveness. Each of the control clones produced rapidly growing, highly metastatic tumors within 2 wk of inoculation on chorioallantoic membranes (CAMs) of chick embryos. In contrast, each of the clones with low surface uPAR, whose proliferation rate in culture was indistinguishable from controls, remained dormant for up to 5 mo when inoculated on CAMs. Thus, the reduction in uPAR altered the phenotype of HEp3 tumor cells from tumorigenic to dormant. Although protracted, tumor dormancy was not permanent since in spite of maintaining low uPAR levels, each of the in vivo-passaged antisense clones eventually reemerged from dormancy to initiate progressive growth and to form metastases at a level of 20 to 90% of that of fully malignant control. This observation suggested that other factors, whose expression is dependent on cumulative and prolonged in vivo effects, can compensate for the lack of a full complement of surface uPAR required for the expression of malignant properties. These "reemerged," uPAR-deficient clones were easily distinguishable from the vector-transfected controls by the fact that after only 1 wk in culture, the invasion of CAM by all five clones and tumorigenicity of four of the five clones were reduced back to the values observed before in vivo maintenance. In contrast, dissociated and in vitro-grown cells of control tumors were fully invasive and produced large, metastatic tumors when reinoculated on CAMs. Quantitation of the percent of apoptotic and S-phase cells in vivo, in the control and uPAR-deficient, dormant clones, showed that the mechanism responsible for the dormancy was a diminished proliferation.
Figures
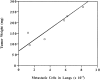
Figure 7
Dependence of LK 25 metastasis on primary tumor mass; standard curve. In vivo–grown LK 25 cells were dissociated, kept in culture for 1 wk, and reinoculated on fresh CAMs. After 1 wk on CAM, tumors were excised and weighed. The number of metastatic cells in embryo lungs was measured (see Materials and Methods). As seen, within the range of 90–270 mg, metastasis level is directly proportional to the tumor mass. Tumors smaller than 50 mg do not yield easily detectable metastases.
Figure 1
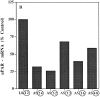
Reduction of uPAR-mRNA level in antisense-transfected clones. uPAR-mRNA steady-state levels were determined in parental HEp3 cells, two control clones, and five uPAR antisense clones by Northern blot hybridization (30 μg total RNA) using uPAR-cDNA (exposure 6 h) and, after stripping, GAPDHcDNA (exposure 1.5 h) as probes. The film was scanned, and the values were expressed as units of uPAR-mRNA per unit of GAPDH-mRNA and calculated as percent of uPAR-mRNA in parental cells.
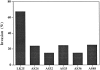
Figure 2
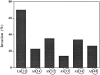
In vitro growth rate of control and antisense clones. Cells (8 × 104) were plated in 35-mm dishes, and two dishes of each clone were trypsinized on four consecutive days and counted. The results shown are calculated by dividing the total number of divisions by 4 d of growth.
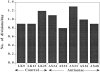
Figure 3
Invasion of “resealed” CAMs by antisense clones grown in culture. 125I-UdR–labeled cells (3 × 105 per CAM) derived from individual clones were inoculated each onto eight CAMs. Before inoculation the CAMs were “wounded” and then allowed to reseal for 24 h. The median of invasion is shown for each group. Comparison of groups by analysis of variance (SYSTAT) analysis showed a highly statistically significant difference (P = 0.000). A post-hoc analysis of each clone versus LK 25 control showed a value of P = 0.000, except for AS 32, in which P = 0.001. Similar results were obtained with control clones (LK 9 and 12) (not shown).
Figure 4
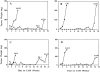
Dormancy of antisense-transfected clones maintained in vivo. Cells of individual clones (5 × 105 per CAM) were inoculated on CAMs of two 10-d-old chick embryos and incubated for 1 wk, and the resulting nodules were excised, weighed, minced, and reinoculated onto two fresh CAMs. Serial passage of tumors were discontinued when their weight exceeded 100 mg. (We observed that tumors of this size, which contain mostly live tumor cells, usually continue progressive growth). M(−) indicates that lungs of these embryos were tested for metastases and found to be negative.
Figure 5
uPAR-mRNA level in cells isolated from reestablished CAM tumors. (A) CAM tumors were dissociated into single-cell suspension, plated, and kept in culture for 1–2 wk. Total RNA was extracted from ∼1 × 107 cells and analyzed by Northern blot hybridization exactly as described in the legend to Fig. 1_._ Film was exposed for 40 min after hybridization with labeled uPAR-cDNA and for 20 min after GAPDH probes. (B) Units calculated from a densitometry scan and expressed as described in the legend to Fig. 1. Only clones AS 32 and 36 had detectable antisense RNA, both when grown in culture and after forming CAM tumors. Circled numbers indicate cells after in vivo growth.
Figure 5
uPAR-mRNA level in cells isolated from reestablished CAM tumors. (A) CAM tumors were dissociated into single-cell suspension, plated, and kept in culture for 1–2 wk. Total RNA was extracted from ∼1 × 107 cells and analyzed by Northern blot hybridization exactly as described in the legend to Fig. 1_._ Film was exposed for 40 min after hybridization with labeled uPAR-cDNA and for 20 min after GAPDH probes. (B) Units calculated from a densitometry scan and expressed as described in the legend to Fig. 1. Only clones AS 32 and 36 had detectable antisense RNA, both when grown in culture and after forming CAM tumors. Circled numbers indicate cells after in vivo growth.
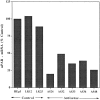
Figure 6
Invasion of “resealed” CAMs by cells isolated from CAM tumors. Cells isolated from progressively growing CAM tumors (LK 25 control, week 4 in vivo, antisense clones weeks 17 to 22 in vivo) were kept in culture for 1 wk, detached, and inoculated on CAMs resealed for 28 h. Invasion was quantitated as described in the legend to Fig. 3. Analysis of variance revealed a significant P = 0.036 difference between the groups. Circled numbers indicate cells after in vivo growth.
Figure 8
Summary of in vitro and in vivo properties of control and uPAR antisense clones. Cells of control clones rapidly form large tumor masses on CAMs (large open spheres), while cells in which uPAR is inhibited remain dormant in vivo (small circles) for as long as 5 mo and then reemerge to form tumors. However, while the tumorigenic and metastatic properties of the control clones are stable, the compensatory mechanism operating in vivo and leading to the interruption of dormancy produces heterogeneous and unstable phenotype that never reaches the full malignant potential of the controls. H, high; I, intermediate; L, low.
Similar articles
- The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy.
Kook YH, Adamski J, Zelent A, Ossowski L. Kook YH, et al. EMBO J. 1994 Sep 1;13(17):3983-91. doi: 10.1002/j.1460-2075.1994.tb06714.x. EMBO J. 1994. PMID: 8076594 Free PMC article. - Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling.
Aguirre Ghiso JA, Kovalski K, Ossowski L. Aguirre Ghiso JA, et al. J Cell Biol. 1999 Oct 4;147(1):89-104. doi: 10.1083/jcb.147.1.89. J Cell Biol. 1999. PMID: 10508858 Free PMC article. - In vitro inhibition of human glioblastoma cell line invasiveness by antisense uPA receptor.
Mohanam S, Chintala SK, Go Y, Bhattacharya A, Venkaiah B, Boyd D, Gokaslan ZL, Sawaya R, Rao JS. Mohanam S, et al. Oncogene. 1997 Mar 20;14(11):1351-9. doi: 10.1038/sj.onc.1200963. Oncogene. 1997. PMID: 9178895 - Inhibition of the tumor-associated urokinase-type plasminogen activation system: effects of high-level synthesis of soluble urokinase receptor in ovarian and breast cancer cells in vitro and in vivo.
Magdolen V, Krüger A, Sato S, Nagel J, Sperl S, Reuning U, Rettenberger P, Magdolen U, Schmitt M. Magdolen V, et al. Recent Results Cancer Res. 2003;162:43-63. doi: 10.1007/978-3-642-59349-9_4. Recent Results Cancer Res. 2003. PMID: 12790320 Review.
Cited by
- The dormancy dilemma: quiescence versus balanced proliferation.
Wells A, Griffith L, Wells JZ, Taylor DP. Wells A, et al. Cancer Res. 2013 Jul 1;73(13):3811-6. doi: 10.1158/0008-5472.CAN-13-0356. Epub 2013 Jun 21. Cancer Res. 2013. PMID: 23794703 Free PMC article. Review. - Immunohistochemical detection of high-mobility group box 1 correlates with resistance of preoperative chemoradiotherapy for lower rectal cancer: a retrospective study.
Hongo K, Kazama S, Tsuno NH, Ishihara S, Sunami E, Kitayama J, Watanabe T. Hongo K, et al. World J Surg Oncol. 2015 Jan 27;13:7. doi: 10.1186/1477-7819-13-7. World J Surg Oncol. 2015. PMID: 25622595 Free PMC article. - Clinical opportunities and challenges in targeting tumour dormancy.
Hensel JA, Flaig TW, Theodorescu D. Hensel JA, et al. Nat Rev Clin Oncol. 2013 Jan;10(1):41-51. doi: 10.1038/nrclinonc.2012.207. Epub 2012 Nov 27. Nat Rev Clin Oncol. 2013. PMID: 23183631 Review. - Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists.
Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, Galanos C, André F, Kroemer G, Zitvogel L. Yamazaki T, et al. Cell Death Differ. 2014 Jan;21(1):69-78. doi: 10.1038/cdd.2013.72. Epub 2013 Jun 28. Cell Death Differ. 2014. PMID: 23811849 Free PMC article. - The effect of suramin on the resorption of bovine nasal cartilage.
Lewis CL, Frazer A, Russell RG, Bunning RA. Lewis CL, et al. Inflammopharmacology. 1999;7(4):387-400. doi: 10.1007/s10787-999-0032-x. Inflammopharmacology. 1999. PMID: 17657441
References
- Bianchi E, Cohen RL, Thor AT, Todd RF, III, Mizukami IF, Lawrence DA, Ljung BM, Shuman MA, Smith HS. The urokinase receptor is expressed in invasive breast cancer but not in normal breast tissue. Cancer Res. 1994;54:861–866. - PubMed
- Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. BioEssays. 1993;15:105–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous