Identification of endogenous sympathetic neuron pituitary adenylate cyclase-activating polypeptide (PACAP): depolarization regulates production and secretion through induction of multiple propeptide transcripts - PubMed (original) (raw)
Identification of endogenous sympathetic neuron pituitary adenylate cyclase-activating polypeptide (PACAP): depolarization regulates production and secretion through induction of multiple propeptide transcripts
C A Brandenburg et al. J Neurosci. 1997.
Abstract
The vasoactive intestinal peptide/pituitary adenylate cyclase-activating polypeptide (PACAP)/secretin/glucagon family of peptides displays numerous physiological roles in autonomic nervous system development and function. The regulated endogenous production and release of PACAP peptides in sympathetic neurons of the superior cervical ganglion (SCG) was investigated. The two posttranslationally processed forms of PACAP, PACAP27 and PACAP38, were identified in rat adult, neonatal, and cultured SCG neurons. PACAP38 levels were approximately 5-10 fmol/adult SCG and approximately 2 fmol/neonatal SCG; PACAP27 levels were comparable. The authenticity of peptide immunoreactivity in these tissues was verified by coelution with synthetic PACAP in reverse-phase HPLC analysis. Reverse transcription-PCR and sequence-specific hybridization revealed PACAP mRNA in adult, neonatal, and cultured SCG neurons; in situ hybridization histochemistry and immunocytochemistry localized the PACAP peptide and proPACAP mRNA to a subset of the SCG neuronal population. Basal and stimulated release of endogenous PACAP38 from cultured sympathetic neurons was established, suggesting that these peptides may function as signaling molecules at target tissues. Chronic depolarization with 40 mM potassium stimulated the PACAP secretory rate 10- to 20-fold, with concomitant increases in cellular PACAP peptide and mRNA levels. When examined using Northern analysis, depolarizing conditions not only stimulated the 2.2 kb form of PACAP mRNA, but also induced the expression of a shortened, 0.9 kb, transcript. Further reverse-transcription PCR analysis demonstrated that this smaller transcript was not identical to the unique testicular message. These studies identify PACAP38 and PACAP27 as regulated endogenous releasable peptides contributing to the functional diversity and phenotypic plasticity of the sympathetic nervous system.
Figures
Fig. 9.
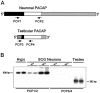
Reverse transcription-PCR and gene-specific hybridization reveal that the smaller pro-PACAP transcript induced in SCG neurons is not the testicular form of the message.A, Schematic representation of the neuronal and testicular pro-PACAP transcripts demonstrating the positions of the transcript-specific oligonucleotide primer pairs; the neuronal pro-PACAP transcript-specific primers are PCP1 and PCP2 and the testicular pro-PACAP transcript-specific primers are PCP3 and PCP4 (Table 1). White, coding region; black, 5′ untranslated region; gray, 3′-untranslated region;hatched, testes-specific 5′-untranslated region.B, Total RNA from individual SCG neuronal cultures incubated in medium containing 40 m
m
NaCl (−K+) or 40 m
m
KCl (+K+) was reverse-transcribed, and the cDNA was amplified using the neuronal transcript-specific primers (PCP1 and PCP2) or the testicular transcript-specific primers (PCP3 and_PCP4_). Total hypothalamic (Hypo) and testicular (Testes) RNA were reverse-transcribed and amplified with the neuronal and testicular primer pairs, respectively. The amplified products were fractionated, blotted, and hybridized with a radiolabeled synthetic internal oligonucleotide probe located within the pro-PACAP coding region that is identical for the neuronal and testicular transcripts; the membranes were apposed to film for 150 min (SCG neurons ± K+, both primer pairs), 70 min (hypothalamus), or 40 min (testes). The predicted product sizes are 606 bp for the neuronal transcript-specific primers and 362 bp for the testicular transcript-specific primers. Representative samples are shown; identical results were obtained from two independent culture preparations consisting of four to six individual sympathetic culture wells for each treatment.
Fig. 1.
Tissues innervated by the SCG differentially express isoforms of the type I PACAP-selective (PACAPR), VIP1/PACAP (VIP1R) and VIP2/PACAP (VIP2R) receptors. Total RNA from individual adult male rat pineal, sublingual, and submaxillary glands; iris; and cerebral blood vessels was reverse-transcribed, and the cDNA was amplified using primers flanking the insertion site of the alternative splice variants of the type I PACAP receptor or primers specific for each of the VIP/PACAP receptors (Table 1). The amplified products and a 100 bp DNA ladder were resolved on 1.6% agarose gels, stained with ethidium bromide, and visualized by UV illumination. The predicted sizes of the products are 303, 387, and 471 bp for the type I PACAP receptor variants containing neither, one, and both 84 bp cassettes, respectively, and 323 and 396 bp for the VIP1/PACAP and VIP2/PACAP receptors.
Fig. 2.
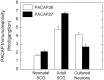
PACAP38 and PACAP27 immunoreactivities are expressed endogenously by SCGs neurons. Individual adult and neonatal (postnatal day 1) rat SCG were extracted for peptide level analysis. Dissociated SCG neurons, plated at an initial density of 1.5 × 104 cells/cm2 into 16 mm dishes (2 cm2), were cultured in defined medium for 9 d and harvested for assay. Endogenous pro-PACAP peptide levels were determined by radioimmunoassay using antisera specific for PACAP38 (open bar) or PACAP27 (filled bar). Data represent the mean femtomoles of PACAP immunoreactivity per ganglion or ganglion equivalent (26,000 cultured neurons) ± SEM (n = 10–12 for each preparation and peptide).
Fig. 3.
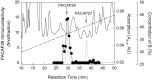
SCG PACAP immunoreactivity represents authentic peptide. Adult SCG extracts were analyzed by combined reverse-phase HPLC and radioimmunoassay. Samples were fractionated on a μRPC 3.2/3 C2/C18 column using a TFA/acetonitrile gradient solvent system as described in Materials and Methods. At a flow rate of 50 μl/min, 25 μl fractions were collected. The fractions subsequently were processed and assayed for PACAP38 immunoreactivity. Solid line, UV absorbance at 214 nm of SCG extract; dotted line, UV elution profile of synthetic PACAP38 and PACAP27 (300 ng each); •—•, SCG extract PACAP38 immunoreactivity (fmol/fraction); dashed line, concentration gradient of solvent B (0.1% TFA/80% acetonitrile).
Fig. 4.
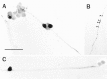
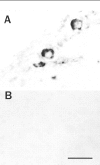
PACAP immunoreactivity is localized to a small population of sympathetic neurons in vitro. SCG neurons, cultured for 9 d, were fixed with 4% paraformaldehyde and stained immunocytochemically with 1:10,000 anti-PACAP38 using the avidin–biotin–peroxidase complex technique (A_–_C). A subpopulation of neurons was stained heterogeneously, and PACAP38 immunoreactivity was localized to the processes, varicosities (arrowheads), and soma of a distinct population of neurons. Scale bar, 100 μm.
Fig. 5.
Sympathetic neurons express pro-PACAP mRNA. Total RNA from individual adult and neonatal SCGs, and sympathetic neuronal cultures (2 cm2 wells) was reverse-transcribed, and the cDNA was amplified for 35 cycles using primers PCP1 and PCP2 (Table 1), which are specific for the rat neuronal pro-PACAP transcript. The identity of the neuronal pro-PACAP reverse transcription-PCR products was verified using sequence-specific hybridization. For product identification, the amplified products were transferred to a nylon membrane and hybridized with an internal oligonucleotide end-labeled with [γ32P]-ATP. The predicted 606 bp product identified by gene-specific probe hybridization of the amplified products is indicated.
Fig. 6.
PACAP mRNA is localized to a subpopulation of sympathetic neurons in vivo. In situ hybridization histochemistry using antisense (A) and sense (B) digoxigenin-labeled PACAP riboprobes was performed on adult male SCG cryosections as described in Materials and Methods. After incubation with antidigoxigenin alkaline phosphatase, sections were processed with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate as phosphatase substrates. Scale bar, 50 μm.
Fig. 7.
Depolarization stimulates sympathetic neuron PACAP release. SCG neurons were dissociated enzymatically and plated at an initial density of 1.5 × 104 cells/cm2onto 16 mm (2 cm2) dishes. On day 9 of culture, the sympathetic neurons were incubated in 500 μl of defined medium containing 40 m
m
NaCl (control, ▪) or 40 m
m
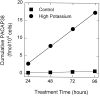
KCl (high potassium, •) for 96 hr. Every 24 hr, the conditioned medium was collected for PACAP38 radioimmunoassay, and replaced with fresh medium containing NaCl or KCl. Data represent the mean cumulative femtomoles of PACAP38 immunoreactivity per 104 cells ± SEM (n = 3–6 samples). Error bars are within the symbols.
Fig. 8.
Depolarization induces multiple pro-PACAP mRNA levels. Regulation of cellular pro-PACAP mRNA expression was evaluated by Northern blot analysis. SCG neurons were incubated in medium containing 40 m
m
NaCl (−K+) or 40 m
m
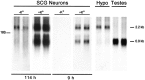
KCl (+K+) for 96 hr beginning on day 9 of culture. Poly(A+) RNA was isolated from total RNA from 1.8 × 105 sympathetic neurons, separated on denaturing gels, transferred to a nylon membrane, and hybridized to a pro-PACAP-specific radiolabeled riboprobe. Representative autoradiograms are shown (n = 4). Hybridization to the pro-PACAP transcripts was examined at shorter (9 hr) and longer (114 hr) film exposure times. Two micrograms of poly(A+) mRNA from hypothalamus (Hypo) and testes were analyzed for pro-PACAP mRNA in parallel and exposed to film for 39 hr.
Similar articles
- Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptide Y and catecholamine expression.
May V, Braas KM. May V, et al. J Neurochem. 1995 Sep;65(3):978-87. doi: 10.1046/j.1471-4159.1995.65030978.x. J Neurochem. 1995. PMID: 7643128 - Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia.
Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Braas KM, et al. J Neurosci. 1998 Dec 1;18(23):9766-79. doi: 10.1523/JNEUROSCI.18-23-09766.1998. J Neurosci. 1998. PMID: 9822736 Free PMC article. - Mechanisms of pituitary adenylate cyclase activating polypeptide (PACAP)-induced depolarization of sympathetic superior cervical ganglion (SCG) neurons.
May V, Beaudet MM, Parsons RL, Hardwick JC, Gauthier EA, Durda JP, Braas KM. May V, et al. Ann N Y Acad Sci. 1998 Dec 11;865:164-75. doi: 10.1111/j.1749-6632.1998.tb11175.x. Ann N Y Acad Sci. 1998. PMID: 9928009 Review.
Cited by
- Pituitary adenylate cyclase-activating polypeptide (PACAP) alters parasympathetic neuron gene expression in a time-dependent fashion.
Sumner AD, Margiotta JF. Sumner AD, et al. J Mol Neurosci. 2008 Nov;36(1-3):141-56. doi: 10.1007/s12031-008-9103-5. Epub 2008 Jul 2. J Mol Neurosci. 2008. PMID: 18594777 - Microarray analyses of pituitary adenylate cyclase activating polypeptide (PACAP)-regulated gene targets in sympathetic neurons.
Braas KM, Schutz KC, Bond JP, Vizzard MA, Girard BM, May V. Braas KM, et al. Peptides. 2007 Sep;28(9):1856-70. doi: 10.1016/j.peptides.2007.04.004. Epub 2007 Apr 19. Peptides. 2007. PMID: 17512639 Free PMC article. - PACAP signaling in stress: insights from the chromaffin cell.
Eiden LE, Emery AC, Zhang L, Smith CB. Eiden LE, et al. Pflugers Arch. 2018 Jan;470(1):79-88. doi: 10.1007/s00424-017-2062-3. Epub 2017 Sep 30. Pflugers Arch. 2018. PMID: 28965274 Free PMC article. Review. - Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling.
Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Amburgey OA, et al. Hypertension. 2010 Nov;56(5):1003-8. doi: 10.1161/HYPERTENSIONAHA.110.158931. Epub 2010 Sep 20. Hypertension. 2010. PMID: 20855653 Free PMC article. - Circulating PACAP peptide and PAC1R genotype as possible transdiagnostic biomarkers for anxiety disorders in women: a preliminary study.
Ross RA, Hoeppner SS, Hellberg SN, O'Day EB, Rosencrans PL, Ressler KJ, May V, Simon NM. Ross RA, et al. Neuropsychopharmacology. 2020 Jun;45(7):1125-1133. doi: 10.1038/s41386-020-0604-4. Epub 2020 Jan 7. Neuropsychopharmacology. 2020. PMID: 31910434 Free PMC article.
References
- Arimura A. Receptors for pituitary adenylate cyclase-activating polypeptide: comparison with vasoactive intestinal peptide receptors. Trends Endocrinol Metab. 1992;3:288–294. - PubMed
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. - PubMed
- Beaudet M, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic neurons innervating the superior cervical ganglion. Soc Neurosci Abstr. 1996;22:1998. - PubMed
- Bennett HPJ, Browne CA, Solomon CA. Complete purification of pituitary peptides using reverse-phase HPLC alone. In: Rich DH, Gross E, editors. Peptides. Pierce Chemical; Rockford, IL: 1981. pp. 785–788.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources