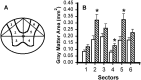Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury - PubMed (original) (raw)
Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury
Y D Teng et al. J Neurosci. 1997.
Abstract
Although relatively little is known of the mechanisms involved in secondary axonal loss after spinal cord injury (SCI), recent data from in vitro models of white matter (WM) injury have implicated abnormal sodium influx as a key event. We hypothesized that blockade of sodium channels after SCI would reduce WM loss and long-term functional deficits. To test this hypothesis, a sufficient and safe dose (0.15 nmol) of the potent Na+ channel blocker tetrodotoxin (TTX) was determined through a dose-response study. We microinjected TTX or vehicle (VEH) into the injury site at 15 min after a standardized contusive SCI in the rat. Behavioral tests were performed 1 d after injury and weekly thereafter. Quantitative histopathology at 8 weeks postinjury showed that TTX treatment significantly reduced tissue loss at the injury site, with greater effect on sparing of WM than gray matter. TTX did not change the pattern of chronic histopathology typical of this SCI model, but restricted its extent, tripled the area of residual WM at the epicenter, and reduced the average length of the lesions. Serotonin immunoreactivity caudal to the epicenter, a marker for descending motor control axons, was nearly threefold that of VEH controls. The increase in WM at the epicenter was significantly correlated with the decrease in functional deficits. The TTX group exhibited a significantly enhanced recovery of coordinated hindlimb functions, more normal hindlimb reflexes, and earlier establishment of a reflex bladder. The results demonstrate that Na+ channels play a critical role in WM loss in vivo after SCI.
Figures
Fig. 1.
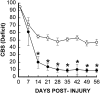
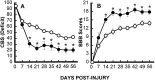
Effect of TTX on overall hindlimb deficits over time after SCI. Deficits are expressed as a combined behavioral score (CBS) (Gale et al., 1985). Data points represent the average CBS per group where groups received either VEH alone (○,n = 4) or 1.0 nmol TTX (•, _n_= 5). Data were analyzed with repeated measures ANOVA, which showed an overall significant (p < 0.05) effect of treatment. Asterisks indicate that means are significantly different from the VEH-treated control group at the specified times after SCI (Tukey’s procedure).
Fig. 2.
Effect of TTX (0.15 nmol) on recovery of hindlimb reflexes. A, Effect of TTX on recovery of the reflex to withdraw the hindlimb in response to pressure applied to the toe pads (pressure withdrawal reflex). B, Effects of TTX on recovery of the righting reflex to turn over in response to abnormal body position (righting reflex). Data points represent the percentage of rats in each group (n = 12 per group; VEH, ○; TTX, •) that exhibit a normal reflex.
Fig. 3.
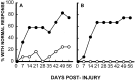
Effect of TTX on recovery of coordinated hindlimb functions. Comparison of groups (n = 12) that received VEH alone (○) or TTX (•). A, Percentage of rats that use their hindlimbs in swimming. B, Inclined plane performance. Data points represent average ± SEM maximum angle at which rats can maintain position for 5 sec. Data were analyzed with repeated measures ANOVA, which showed an overall significant (p < 0.05) effect of treatment.Asterisks indicate that means are significantly different from the control group at the specified times after SCI (Tukey’s procedure).
Fig. 4.
Effect of TTX on the recovery of locomotor function after SCI. Open-field locomotion was observed and graded on a 0–5 scale (the motor score) (Wrathall et al., 1985), in which a grade of 3 or more indicates the ability to bear weight and use the hindlimbs for effective locomotion. Data points are individual scores for rats that received TTX (•) or VEH alone (○) (n = 12 per group). The bars represent the modal score for the group. The extent of recovery as measured by the motor scores was significantly (p < 0.05) higher in the TTX group at 14, 21, 28, and 56 d p.i. (Wilcoxon scores for rank sums).
Fig. 5.
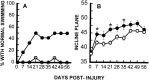
Hindlimb function over time after SCI in groups (n = 12) that received either VEH alone (○) or 0.15 nmol TTX (•). A, Overall functional deficits expressed as the combined behavioral score (CBS) (Gale et al., 1985). B, Locomotor function graded on an expanded scale (BBB) (Basso et al., 1995) that ranges from 21 in normal rats to 0 in rats with complete hindlimb paralysis. Data points represent the group average. Where no error bar is shown, the SEM was smaller than the symbol. Data were analyzed with repeated measures ANOVA, which showed an overall significant (p < 0.05) effect of treatment.Asterisks indicate that means are significantly different from the VEH-treated control group at the specified times after SCI (Tukey’s procedure).
Fig. 6.
Effect of TTX on residual spinal cord tissue at 8 weeks after injury. Tracing of sections through the lesion epicenters in rats of the TTX-treated group (A) and VEH-treated control group (C). Epicenters from the TTX-treated group usually show a complete thin rim of peripheral myelinated and hypomyelinated WM (not distinguished in these tracings), and in occasional cases, peripheral portions of dorsal horn GM (cross-hatched). The centers of the lesions contain cavities and a loose network of lesion cells (stippled). Also shown are three-dimensional reconstructions of spinal cord of two individual rats representative (with final CBS similar to the group mean) of the TTX-treated group (B) and the VEH-treated group (D). Color code: red for WM;orange for GM; dark blue for hypomyelinated WM; blue for lesion cells; and_pink_ for cavity.
Fig. 7.
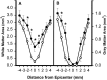
Effect of TTX on lesion morphometry at 2 months after injury. The area of spared WM (A) and GM (B) in spinal cord sections caudal (−1 to −4 mm) and rostral (1–4 mm) to the injury epicenter. Data represent the average from 12 rats per group. Groups received VEH alone (○) or 0.15 nmol TTX (•). There was a significant overall difference (p < 0.05) between the groups (repeated measures ANOVA). Asterisks indicate that means are significantly different from the VEH-treated group at the specified locations (Tukey’s procedure).
Fig. 8.
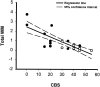
Linear regression analysis. A significant correlation between sparing of total WM (myelinated + hypomyelinated WM) at the lesion epicenter and reduction of functional deficits as estimated by the CBS at 8 weeks after SCI. r = 0.796; _R_2 = 0.634; p < 0.001 (ANOVA). Analysis was based on 12 rats from each group that received VEH alone (○) or 0.15 nmol TTX (•).
Fig. 9.
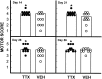
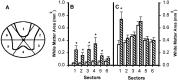
Effect of microinjected TTX on residual WM in different radial sectors of the spinal cord at 8 weeks after injury.A, WM sectors that were analyzed. B, Sector analysis of sections at the lesion epicenter in groups of rats microinjected with VEH alone (open bars) or 0.15 nmol TTX (hatched bars) at 15 min after SCI (n = 12 per group). C, Analysis of sectors at 3 mm caudal to the epicenter. Repeated measures ANOVA indicates an overall significant difference (p < 0.05). _Asterisks_signify that means are significantly different from the VEH-treated group in the specified sectors (Tukey’s procedure).
Fig. 10.
Effect of microinjected TTX on residual GM in different sectors of the spinal cord at 8 weeks after injury.A, GM sectors that were analyzed. B, GM sector analysis of sections at 3 mm caudal to the lesion epicenter in groups of rats microinjected with VEH alone (open bars) or 0.15 nmol TTX (hatched bars) at 15 min after SCI (n = 12 per group). An overall significant difference (p < 0.05) was found between the groups. Asterisks indicate that means are significantly different from the VEH-treated group in the specified sectors (Tukey’s procedure).
Similar articles
- Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury.
Rosenberg LJ, Teng YD, Wrathall JR. Rosenberg LJ, et al. J Neurosci. 1999 Jul 15;19(14):6122-33. doi: 10.1523/JNEUROSCI.19-14-06122.1999. J Neurosci. 1999. PMID: 10407048 Free PMC article. - Time course studies on the effectiveness of tetrodotoxin in reducing consequences of spinal cord contusion.
Rosenberg LJ, Wrathall JR. Rosenberg LJ, et al. J Neurosci Res. 2001 Oct 15;66(2):191-202. doi: 10.1002/jnr.1211. J Neurosci Res. 2001. PMID: 11592114 - Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma.
Wrathall JR, Teng YD, Marriott R. Wrathall JR, et al. Exp Neurol. 1997 Jun;145(2 Pt 1):565-73. doi: 10.1006/exnr.1997.6506. Exp Neurol. 1997. PMID: 9217092 - Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX.
Wrathall JR, Choiniere D, Teng YD. Wrathall JR, et al. J Neurosci. 1994 Nov;14(11 Pt 1):6598-607. doi: 10.1523/JNEUROSCI.14-11-06598.1994. J Neurosci. 1994. PMID: 7965063 Free PMC article.
Cited by
- Topiramate treatment is neuroprotective and reduces oligodendrocyte loss after cervical spinal cord injury.
Gensel JC, Tovar CA, Bresnahan JC, Beattie MS. Gensel JC, et al. PLoS One. 2012;7(3):e33519. doi: 10.1371/journal.pone.0033519. Epub 2012 Mar 13. PLoS One. 2012. PMID: 22428066 Free PMC article. - Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury.
Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, Noble-Haeusslein LJ. Hsu JY, et al. J Neurosci. 2006 Sep 27;26(39):9841-50. doi: 10.1523/JNEUROSCI.1993-06.2006. J Neurosci. 2006. PMID: 17005848 Free PMC article. - Cytoplasmic extracts from adipose tissue stromal cells alleviates secondary damage by modulating apoptosis and promotes functional recovery following spinal cord injury.
Kang SK, Yeo JE, Kang KS, Phinney DG. Kang SK, et al. Brain Pathol. 2007 Jul;17(3):263-75. doi: 10.1111/j.1750-3639.2007.00070.x. Epub 2007 Apr 23. Brain Pathol. 2007. PMID: 17465991 Free PMC article. - Transplantation of neural progenitor cells in chronic spinal cord injury.
Jin Y, Bouyer J, Shumsky JS, Haas C, Fischer I. Jin Y, et al. Neuroscience. 2016 Apr 21;320:69-82. doi: 10.1016/j.neuroscience.2016.01.066. Epub 2016 Feb 4. Neuroscience. 2016. PMID: 26852702 Free PMC article. - Assessment of white matter loss using bond-selective photoacoustic imaging in a rat model of contusive spinal cord injury.
Wu W, Wang P, Cheng JX, Xu XM. Wu W, et al. J Neurotrauma. 2014 Dec 15;31(24):1998-2002. doi: 10.1089/neu.2014.3349. Epub 2014 Sep 26. J Neurotrauma. 2014. PMID: 24850066 Free PMC article.
References
- Allen AR. Remarks on histopathological changes in spinal cord due to impact: an experimental study. J Nerv Ment Dis. 1914;41:141–147.
- Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978a;39:236–253. - PubMed
- Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978b;39:254–266. - PubMed
- Balentine JD, Spector M. Calcification of axons in spinal cord trauma. Ann Neurol. 1977;2:520–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous