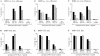Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response - PubMed (original) (raw)
Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response
N M Steven et al. J Exp Med. 1997.
Abstract
Epstein-Barr virus (EBV), a human gamma-herpesvirus, can establish both nonproductive (latent) and productive (lytic) infections. Although the CD8+ cytotoxic T lymphocyte (CTL) response to latently infected cells is well characterized, very little is known about T cell controls over lytic infection; this imbalance in our understanding belies the importance of virus-replicative lesions in several aspects of EBV disease pathogenesis. The present work shows that the primary CD8+ CTL response to EBV in infectious mononucleosis patients contains multiple lytic antigen-specific reactivities at levels at least as high as those seen against latent antigens; similar reactivities are also detectable in CTL memory. Clonal analysis revealed individual responses to the two immediate early proteins BZLF1 and BRLF1, and to three (BMLF1, BMRF1, and BALF2) of the six early proteins tested. In several cases, the peptide epitope and HLA-restricting determinant recognized by these CTLs has been defined, one unusual feature being the number of responses restricted through HLA-C alleles. The work strongly suggests that EBV-replicative lesions are subject to direct CTL control in vivo and that immediate early and early proteins are frequently the immunodominant targets. This contrasts with findings in alpha- and beta-herpesvirus systems (herpes simplex, cytomegalovirus) where viral interference with the antigen-processing pathway during lytic infection renders immediate early and early proteins much less immunogenic. The unique capacity of gamma-herpesvirus to amplify the viral load in vivo through a latent growth-transforming infection may have rendered these agents less dependent upon viral replication as a means of successfully colonizing their hosts.
Figures
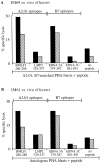
Figure 1
Screening of IM primary effectors immediately ex vivo (A–C) and after LCL stimulation in vitro and expansion to a polyclonal CTL line (D–F). Epitope-specific reactivities were detected using autologous PHA blast targets pretreated with the denoted epitope peptides at 2 μg/ml or with an equivalent concentration of DMSO solvent alone (“no peptide” control). Results are expressed as percentage specific lysis in 7-h chromium release assays for (A) IM53 effectors tested ex vivo at E/T ratios of 60:1 (▪) and 30:1 (▨ ); (B) IM59 effectors tested ex vivo at E/T ratios of 80:1 (▪), 40:1 (▨ ), and 20:1 (▧ ); (C) IM63 effectors tested ex vivo at E/T ratios of 70:1 (▪), 30:1 (▨ ), and 15:1 (▧ ); (D) IM53 CTL line at E/T ratios of 10:1 (▪) and 5:1 (▨ ); (E) IM59 CTL line at E/T ratios of 10:1 (▪) and 5:1 (▨ ); and (F) IM63 CTL line at E/T ratios of 20:1 (▪) and 10:1 (▨ ).
Figure 2
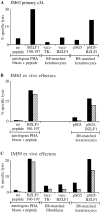
Screening of BZLF1 190-197 epitope-specific effectors for recognition of targets expressing the BZLF1 protein. Assays were conducted on autologous PHA blast targets pretreated with the BZLF1 190197 peptide at 2 μg/ml or with an equivalent concentration of DMSO alone (“no peptide” control), and on HLA-B8–matched keratinocyte or fibroblast targets infected with vacc-BZLF1 or with vacc-TK− as a control, or transiently transfected with pSG5-BZLF1 or with pSG5 as a control. Results are expressed as in Fig. 1 for (A) IM63 primary clone 34 established by limiting dilution cloning of IM63 effectors ex vivo and tested at a E/T ratio of 5:1, and for (B) IM63 ex vivo effectors and (C) IM59 ex vivo effectors, both tested at E/T ratios of 80:1 (▪) and 40:1 (▨ ).
Figure 3
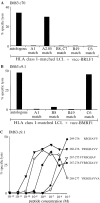
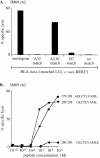
Cytotoxicity testing of CTL clones established by limiting dilution cloning of IM63 effectors ex vivo. Analysis of (A) IM63 primary clone 70 and (B) IM63 primary clone 9.1 for HLA restriction by screening on the autologous LCL and on a panel of allogeneic LCLs infected with (A) vacc-BMLF1 and (B) vacc-BMRF1. For each allogeneic LCL target, the HLA class I alleles shared with IM63 are indicated; note that in (A) the A1- and the C6-matched targets also express HLA-A2.01, indicating that this A2.05-restricted CTL clone cannot use A2.01 as a restriction element. (C) Analysis of IM63 primary clone 9.1 for peptide epitope specificity by screening on autologous PHA blast targets pretreated with the following BMRF1 peptides at 10−5 to 10−12 M concentrations; BMRF1 267-276 (♦), 267-275 (▿), 268-277 (⋄), 268-276 (▾), and 269-276 (•). Results are expressed as in Fig. 1 and all assays were conducted at an E/T ratio of 5:1.
Figure 4
Cytotoxicity testing of IM69 primary clone 62 established by limiting dilution cloning of IM69 effectors ex vivo. (A) Analysis of HLA restriction by screening on the vacc-BMLF1–infected autologous LCL and on a panel of vacc-BMLF1–infected allogeneic LCLs sharing individual HLA class I alleles with IM69 as indicated. (B) Analysis of peptide epitope specificity by screening on autologous PHA blast targets pretreated with the following BMLF1 peptides at 10−5 to 10−10 M concentrations: BMLF1 279-288 (•), 280-288 (▾), and 280-289 (♦). Results are expressed as in Fig. 1 and all assays were conducted at an E/T ratio of 5:1.
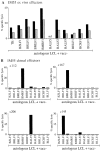
Figure 5
Screening of (A) IM69 and (B) IM61 primary effectors immediately ex vivo for evidence of cytotoxicity against the HLA-A2.01– restricted epitope BMLF1 280-288. Assays were conducted on autologous or on HLA-A2.01, B7-matched PHA blast targets pretreated with the BMLF1 280-288 peptide, with another A2.01-restricted epitope peptide LMP2 329-337, with the B7-restricted epitope peptides EBNA3A 379-387 and EBNA3C 881-891, or with DMSO alone as a “no peptide” control. Results are expressed as in Fig. 1 and both assays were conducted at E/T ratios of 100:1 (▪) and 50:1 (▨ ).
Figure 6
Analysis of the lytic antigen-specific primary CTL response in IM55. (A) Primary effectors, depleted of CD16+ NK cells were assayed immediately ex vivo at E/T ratios of 80:1 (▪) and 40:1 (▨ ) against autologous LCL targets infected with vaccinia recombinants expressing individual lytic cycle genes as indicated. (B) CTL clones established by limiting dilution cloning of IM55 effectors immediately ex vivo were likewise tested for lytic antigen specificity at an E/T ratio of 5:1. Results are expressed as in Fig. 1.
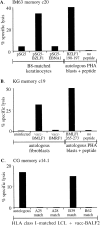
Figure 7
Screening of memory CTL clones for lytic antigen specificity. (A) IM63 memory clone 20, derived from donor IM63 9 mo after recovery from the primary infection, was tested against HLA-B8–matched keratinocyte targets transiently transfected with the pSG5-BZLF1 plasmid or with pSG5 or pSG5 EBNA1 as controls, and against autologous PHA blast targets which had been preexposed to the BZLF1 190-197 peptide at 2 μg/ml or to DMSO alone as a no peptide control. (B) KG memory clone 19, derived from long-term virus carrier KG, was tested against autologous fibroblast targets, either uninfected or infected with the vaccBMLF1 or vacc-BMRF1 recombinants, and against autologous PHA blast targets preexposed to the BMLF1 265-273 peptide at 2 μg/ml or to DMSO alone as a no peptide control. (C) CG memory clone 14.1, derived from long-term virus carrier CG, was tested against the vaccBALF2–infected autologous LCLs and against a range of vacc-BALF2– infected allogeneic LCLs sharing individual HLA class I alleles with donor CG as indicated.
Similar articles
- Selection of a diverse TCR repertoire in response to an Epstein-Barr virus-encoded transactivator protein BZLF1 by CD8+ cytotoxic T lymphocytes during primary and persistent infection.
Silins SL, Cross SM, Elliott SL, Pye SJ, Burrows JM, Moss DJ, Misko IS. Silins SL, et al. Int Immunol. 1997 Nov;9(11):1745-55. doi: 10.1093/intimm/9.11.1745. Int Immunol. 1997. PMID: 9418135 - Dominant cytotoxic T lymphocyte response to the immediate-early trans-activator protein, BZLF1, in persistent type A or B Epstein-Barr virus infection.
Elliott SL, Pye SJ, Schmidt C, Cross SM, Silins SL, Misko IS. Elliott SL, et al. J Infect Dis. 1997 Oct;176(4):1068-72. doi: 10.1086/516513. J Infect Dis. 1997. PMID: 9333169 - Immediate-early transactivator Rta of Epstein-Barr virus (EBV) shows multiple epitopes recognized by EBV-specific cytotoxic T lymphocytes.
Pepperl S, Benninger-Döring G, Modrow S, Wolf H, Jilg W. Pepperl S, et al. J Virol. 1998 Nov;72(11):8644-9. doi: 10.1128/JVI.72.11.8644-8649.1998. J Virol. 1998. PMID: 9765404 Free PMC article. - Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection.
Rickinson AB, Moss DJ. Rickinson AB, et al. Annu Rev Immunol. 1997;15:405-31. doi: 10.1146/annurev.immunol.15.1.405. Annu Rev Immunol. 1997. PMID: 9143694 Review. - Immune surveillance against Epstein-Barr virus.
Moss DJ, Burrows SR, Khanna R, Misko IS, Sculley TB. Moss DJ, et al. Semin Immunol. 1992 Apr;4(2):97-104. Semin Immunol. 1992. PMID: 1377517 Review.
Cited by
- Reconstitution of EBV-directed T cell immunity by adoptive transfer of peptide-stimulated T cells in a patient after allogeneic stem cell transplantation for AITL.
Lammoglia Cobo MF, Ritter J, Gary R, Seitz V, Mautner J, Aigner M, Völkl S, Schaffer S, Moi S, Seegebarth A, Bruns H, Rösler W, Amann K, Büttner-Herold M, Hennig S, Mackensen A, Hummel M, Moosmann A, Gerbitz A. Lammoglia Cobo MF, et al. PLoS Pathog. 2022 Apr 22;18(4):e1010206. doi: 10.1371/journal.ppat.1010206. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35452490 Free PMC article. - Simultaneous assessment of cytotoxic T lymphocyte responses against multiple viral infections by combined usage of optimal epitope matrices, anti- CD3 mAb T-cell expansion and "RecycleSpot".
Bihl FK, Loggi E, Chisholm JV 3rd, Hewitt HS, Henry LM, Linde C, Suscovich TJ, Wong JT, Frahm N, Andreone P, Brander C. Bihl FK, et al. J Transl Med. 2005 May 11;3(1):20. doi: 10.1186/1479-5876-3-20. J Transl Med. 2005. PMID: 15888204 Free PMC article. - Compartmentalization and transmission of multiple epstein-barr virus strains in asymptomatic carriers.
Sitki-Green D, Covington M, Raab-Traub N. Sitki-Green D, et al. J Virol. 2003 Feb;77(3):1840-7. doi: 10.1128/jvi.77.3.1840-1847.2003. J Virol. 2003. PMID: 12525618 Free PMC article. - Accessing Epstein-Barr virus-specific T-cell memory with peptide-loaded dendritic cells.
Redchenko IV, Rickinson AB. Redchenko IV, et al. J Virol. 1999 Jan;73(1):334-42. doi: 10.1128/JVI.73.1.334-342.1999. J Virol. 1999. PMID: 9847337 Free PMC article. - Adoptive transfer of EBV specific CD8+ T cell clones can transiently control EBV infection in humanized mice.
Antsiferova O, Müller A, Rämer PC, Chijioke O, Chatterjee B, Raykova A, Planas R, Sospedra M, Shumilov A, Tsai MH, Delecluse HJ, Münz C. Antsiferova O, et al. PLoS Pathog. 2014 Aug 28;10(8):e1004333. doi: 10.1371/journal.ppat.1004333. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25165855 Free PMC article.
References
- Rickinson, A.B., and E. Kieff. 1996. Epstein-Barr virus. In Fields Virology. B.N. Fields, D.M. Knipe, and P.M. Howley, editors. Lippincott-Raven Publishers, Philadelphia. 2397– 2446.
- Sixbey JW, Nedrud JG, Raab-Traub N, Hanes RA, Pagano JS. Epstein-Barr virus replication in oropharyngeal epithelial cells. N Engl J Med. 1984;310:1225–1230. - PubMed
- Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype and distribution of latently and/or productively Epstein-Barr virus–infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750. - PubMed
- Yao QY, Ogan P, Rowe M, Wood M, Rickinson AB. Epstein-Barr virus–infected B cells persist in the circulation of acyclovir-treated virus carriers. Int J Cancer. 1989;43:67–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials