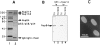Nup84, a novel nucleoporin that is associated with CAN/Nup214 on the cytoplasmic face of the nuclear pore complex - PubMed (original) (raw)
Nup84, a novel nucleoporin that is associated with CAN/Nup214 on the cytoplasmic face of the nuclear pore complex
R Bastos et al. J Cell Biol. 1997.
Abstract
The short filaments extending from the cytoplasmic face of nuclear pore complexes are thought to contain docking sites for nuclear import substrates. One component of these filaments is the large O-linked glycoprotein CAN/Nup214. Immunoprecipitation studies carried out under nondenaturing conditions, and using a variety of antibodies, reveal a novel nonglycosylated nucleoporin, Nup84, that is tightly associated with CAN/Nup214. Consistent with such an association, Nup84 is found to be exposed on the cytoplasmic face of the nuclear pore complex. cDNA sequence analyses indicate that Nup84 contains neither the GLFG nor the XFXFG repeats that are a characteristic of a number of other nuclear pore complex proteins. Secondary structure predictions, however, suggest that Nup84 contains a coiled-coil COOH-terminal domain, a conclusion supported by the observation of significant sequence similarity between this region of the molecule and various members of the tropomyosin family. Mutagenesis and expression studies indicate that the putative coiled-coil domain is required for association with the cytoplasmic face of the nuclear pore complex, whereas it is the NH2-terminal region of Nup84 that contains the site of interaction with CAN/Nup214. These findings suggest a model in which Nup84 may function in the attachment of CAN/Nup214 to the central framework of the nuclear pore complex. In this way, Nup84 could play a central role in the organization of the interface between the pore complex and the cytoplasm.
Figures
Figure 1
(A) Nucleoporins immunoadsorbed from rat liver nuclear envelopes using QE5-Sepharose beads (lane 2). The material shown is derived from 1 to 2 g of rat liver and is revealed by silver staining. Approximately 50– 100× this quantity was used for limited amino acid sequence analysis of Nup84. Molecular weight markers (indicated in kD) are shown in lane 1. (B) Western blot analysis of QE5 and antiNup214 immunoprecipitates of NRK cells using an antipeptide antibody against Nup84. Immunoprecipitates were washed either in a low stringency buffer containing 0.5% Triton X-100 (TX, lanes 1 and 3) that preserves the Nup214–Nup84 interaction, or in a high stringency buffer containing 0.5% Triton X-100 plus 0.1% SDS (TX/SDS, lanes 2 and 4). This latter buffer breaks the interaction between Nup214 and Nup84 (Panté et al., 1994). (C) Indirect immunofluorescence labeling of NRK cells using the anti-peptide antibody against Nup84. The nuclear envelope is clearly labeled.
Figure 2
Complete amino acid sequence of Nup84 derived from cDNA sequence analysis. The boxed segments correspond to the COOH-terminal predicted coiled–coil regions, which are most closely related to members of the tropomyosin family. The primary structure of the underlined peptides was initially elucidated by limited amino acid sequence analysis of Nup84 tryptic fragments. The highlighted peptide (P1), also obtained by amino acid sequence analysis, was used in the design of the oligonucleotide employed in the isolation of the cDNA. The GenBank accession number of the complete cDNA sequence is U93692.
Figure 3
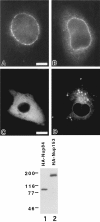
Indirect immunofluorescence analysis of BHK cells expressing HA-Nup84 (A, C) or HA-Nup214 (B, D) at either low (A, B) or high (C, D) levels. The monoclonal antibody used is 12CA5, which recognizes the HA epitope. At low expression levels both proteins localize to the nuclear envelope, whereas at higher levels they spill over into the cytoplasm. At these higher levels their localizations appear quite distinct. While the cytoplasmic distribution of HA-Nup84 is largely uniform, HA-Nup214 tends to concentrate in unusual cytoplasmic foci. The immunoblot is of low stringency QE5 immunoprecipitates of BHK cells expressing either HA-Nup84 (lane 1) or HA-Nup153 (lane 2). The blot is probed with the anti-HA antibody (12CA5). Since HA-Nup84 is not recognized by QE5, its appearance in the immunoprecipitate must be by virtue of its association with CAN/ Nup214. HA-Nup153 (lane 2) provided a positive control for the QE5 immunoprecipitation (the monoclonal antibody QE5 recognizes Nup153 and p62 in addition to CAN/Nup214). Bars: (A, B) 5 μm and (C, D) 10 μm.
Figure 4
Differential permeabilization of BHK cells transiently expressing HA-Nup84. Cells are double labeled with the monoclonal anti-HA (12CA5, A and C) and a rabbit anti-peptide antibody against lamin A (B and D). While 0.2% Triton X-100 (A, B) permeabilizes both the plasma membrane and the nuclear membranes and allows labeling by both antibodies, 0.004% digitonin selectively permeabilizes only the plasma membrane. In this case, HA-Nup84 is detected at the nuclear periphery but lamin A, which is localized on the nucleoplasmic face of the nuclear envelope, remains unlabeled. These results indicate that HA-Nup84 is exposed on the cytoplasmic face of the nuclear envelope. Bar, 10 μm.
Figure 5
Pulse–chase analysis of NPC proteins in BHK cells labeled with 35S-Trans label. The cells were either labeled continuously for 18 h (lanes 7 and 8) or pulse labeled for 30 min (lanes 1–6) followed by a “chase” of 1 h (lanes 3 and 4) or 18 h (lanes 5 and 6) in medium containing excess unlabeled methionine/cysteine. At the appropriate time, the cells were lysed in a low salt buffer containing Triton X-100. The distributions of CAN/Nup214 and Nup84 between soluble and insoluble fractions were then determined by immunoprecipitation analysis using the QE5 monoclonal antibody (which recognizes CAN/Nup214, Nup153, and p62). Total or bulk CAN/Nup214 (and associated Nup84) observed in the samples derived from the continuously labeled cells (lanes 7 and 8) is almost completely (80–90%) resistant to solubilization. In contrast, following the 30-min labeling period, 90% of newly synthesized CAN/Nup214 is found in the soluble fraction (lanes 1 and 2) and, furthermore, coprecipitates with Nup84. Both of these proteins are subsequently “chased” into the insoluble fraction with a half-time of about 1 h (lanes 3 and 4). These findings are consistent with the notion that the two proteins associate before stable integration into the NPC. While derived from the same experiment and run on the same gel, exposure times for lanes 1–6 versus lanes 7 and 8 have been adjusted (11 d and 8 h, respectively) to yield comparable intensities. The two bands indicated by the diamonds, the upper of which migrates just ahead of the p62-associated protein, p58, are both observed with nonimmune Sepharose beads.
Figure 6
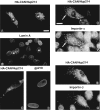
Double indirect immunofluorescence microscopy of BHK cells (A, B, E–H) and HeLa cells (C and D) transiently expressing HACAN/Nup214. Cells were labeled with a monoclonal antibody against the HA epitope (12CA5, A, C, F, and H) as well as rabbit antibodies against lamin A (B), importin-α (F), and importin-β (H) and a human autoantibody recognizing the NPC membrane protein gp210 (C). The same nuclei in A and B are indicated with stars. CAN/Nup214 cytoplasmic foci containing importins-α and -β are indicated with arrows in E–H. While the importins clearly associates with the CAN/Nup214 cytoplasmic foci, these structures are negative for lamin A and gp210. Bars (A–D and E–H) 10 μm.
Figure 7
Double indirect immunofluorescence microscopy of BHKgrβ cells transiently expressing HA-CAN/Nup214. 24 h posttransfection and before fixation, the cells were treated with dexamethasone for periods up to 3 h as indicated at the top of the figure. The primary antibodies were 12CA5 (anti-HA, upper row) and a rabbit anti–β-galactosidase (corresponding fields, lower row). It is evident that there is a transient association of glucocorticoid receptor–β-galactosidase with the CAN/Nup214 foci shortly after the addition of dexamethasone (indicated with arrows in C and D). This is consistent with the observation that importins-α and -β are also associated with these structures (Fig. 6). Bar, 10 μm.
Figure 8
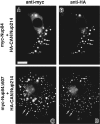
Double indirect immunofluorescence microscopy of BHK cells cotransfected with expression constructs containing either HA-Nup214 and Myc-Nup84 (A and B) or HA-Nup214 and Myc-Nup84Δ607 (C and D). The antibodies used were a monoclonal anti-Myc (9E10, A and C) and a rabbit anti–HA (B and D). It is clear from this figure and from the results presented in Figs. 3 and 9 that there is a codependent distribution of HANup214 and both Myc-Nup84 and Myc-Nup84Δ607. Both Nup84 constructs concentrate in HA-Nup214 foci in the cytoplasm (indicated by the arrows) while there is diminution in the amount of HA-Nup214 localized to the nuclear envelope (compare with Figs. 3 and 5). These results provide strong, albeit circumstantial, evidence that both Myc-Nup84 and Myc-Nup84Δ607 possess the capacity to associate with Nup214. Bar, 10 μm.
Figure 9
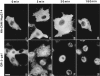
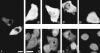
Expression of HA-Nup84Δ607 in BHK cells. HA-Nup84Δ607, detected with 12CA5, localizes exclusively to the cytoplasm regardless of expression level (A). No association with the nuclear envelope is evident. In B–G, the effects of HA-Nup84Δ607 expression (B, D, and F) on endogenous CAN/Nup214 localization (C, E, and G), detected with guinea pig anti–CAN/Nup214 are shown. It is apparent that HA-Nup84Δ607 overexpression causes mislocalization of some of the endogenous CAN/Nup214 to the cytoplasm. For comparison, the effects of full-length HA-Nup84 (H) are also shown. In this case there is no obvious effect on endogenous CAN/Nup214 localization (I). Bars (A and B–I) 10 μm.
Similar articles
- The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm.
Kraemer D, Wozniak RW, Blobel G, Radu A. Kraemer D, et al. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1519-23. doi: 10.1073/pnas.91.4.1519. Proc Natl Acad Sci U S A. 1994. PMID: 8108440 Free PMC article. - The nucleoporin CAN/Nup214 binds to both the cytoplasmic and the nucleoplasmic sides of the nuclear pore complex in overexpressing cells.
Boer JM, van Deursen JM, Croes HJ, Fransen JA, Grosveld GC. Boer JM, et al. Exp Cell Res. 1997 Apr 10;232(1):182-5. doi: 10.1006/excr.1997.3502. Exp Cell Res. 1997. PMID: 9141635 - Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins.
Hu T, Guan T, Gerace L. Hu T, et al. J Cell Biol. 1996 Aug;134(3):589-601. doi: 10.1083/jcb.134.3.589. J Cell Biol. 1996. PMID: 8707840 Free PMC article. - Function and assembly of nuclear pore complex proteins.
Bodoor K, Shaikh S, Enarson P, Chowdhury S, Salina D, Raharjo WH, Burke B. Bodoor K, et al. Biochem Cell Biol. 1999;77(4):321-9. Biochem Cell Biol. 1999. PMID: 10546895 Review. - The nuclear pore complex.
Hurt EC. Hurt EC. FEBS Lett. 1993 Jun 28;325(1-2):76-80. doi: 10.1016/0014-5793(93)81417-x. FEBS Lett. 1993. PMID: 8513897 Review.
Cited by
- The mobile FG nucleoporin Nup98 is a cofactor for Crm1-dependent protein export.
Oka M, Asally M, Yasuda Y, Ogawa Y, Tachibana T, Yoneda Y. Oka M, et al. Mol Biol Cell. 2010 Jun 1;21(11):1885-96. doi: 10.1091/mbc.e09-12-1041. Epub 2010 Apr 7. Mol Biol Cell. 2010. PMID: 20375145 Free PMC article. - Nup153 is an M9-containing mobile nucleoporin with a novel Ran-binding domain.
Nakielny S, Shaikh S, Burke B, Dreyfuss G. Nakielny S, et al. EMBO J. 1999 Apr 1;18(7):1982-95. doi: 10.1093/emboj/18.7.1982. EMBO J. 1999. PMID: 10202161 Free PMC article. - Systematic analysis of barrier-forming FG hydrogels from Xenopus nuclear pore complexes.
Labokha AA, Gradmann S, Frey S, Hülsmann BB, Urlaub H, Baldus M, Görlich D. Labokha AA, et al. EMBO J. 2013 Jan 23;32(2):204-18. doi: 10.1038/emboj.2012.302. Epub 2012 Nov 30. EMBO J. 2013. PMID: 23202855 Free PMC article. - RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase.
Askjaer P, Bachi A, Wilm M, Bischoff FR, Weeks DL, Ogniewski V, Ohno M, Niehrs C, Kjems J, Mattaj IW, Fornerod M. Askjaer P, et al. Mol Cell Biol. 1999 Sep;19(9):6276-85. doi: 10.1128/MCB.19.9.6276. Mol Cell Biol. 1999. PMID: 10454574 Free PMC article. - Moonlighting nuclear pore proteins: tissue-specific nucleoporin function in health and disease.
Jühlen R, Fahrenkrog B. Jühlen R, et al. Histochem Cell Biol. 2018 Dec;150(6):593-605. doi: 10.1007/s00418-018-1748-8. Epub 2018 Oct 25. Histochem Cell Biol. 2018. PMID: 30361777 Review.
References
- Akey CW. Structural plasticity of the nuclear pore complex. J Mol Biol. 1995;248:273–293. - PubMed
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases