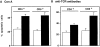Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB - PubMed (original) (raw)
Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB
M R Boothby et al. J Exp Med. 1997.
Abstract
Members of the nuclear factor (NF)-kappaB/Rel family transcription factors are induced during thymic selection and in mature T lymphocytes after ligation of the T cell antigen receptor (TCR). Despite these findings, disruption of individual NF-kappaB/Rel genes has revealed no intrinsic defect in the development of mature T cells, perhaps reflecting functional redundancy. To circumvent this possibility, the T cell lineage was targeted to express a trans-dominant form of IkappaBalpha that constitutively represses the activity of multiple NF-kappaB/Rel proteins. Transgenic cells expressing this inhibitor exhibit a significant proliferative defect, which is not reversed by the addition of exogenous interleukin-2. Moreover, mitogenic stimulation of splenocytes leads to increased apoptosis of transgenic T cells as compared with controls. In addition to deregulated T cell growth and survival, transgene expression impairs the development of normal T cell populations as evidenced by diminished numbers of TCRhi CD8 single-positive thymocytes. This defect was significantly amplified in the periphery and was accompanied by a decrease in CD4(+) T cells. Taken together, these in vivo findings indicate that the NF-kappaB/Rel signaling pathway contains compensatory components that are essential for the establishment of normal T cell subsets.
Figures
Figure 1
Abrogated expression of NF-κB/Rel in IκBα(ΔN) transgenic mice. (A) Transgeneencoded IκBα expressed in thymocytes and splenocytes. Cytoplasmic extracts from unfractionated cell suspensions were subjected to immunopurification using immobilized anti-FLAG M2 antibodies and resolved by SDSPAGE. Resolved proteins were transferred to polyvinylidene difluoride membranes and probed with an antiserum directed against human IκBα (amino acids 289–317) in conjunction with an enhanced chemiluminescence detection system (Amersham Corp., Arlington Heights, IL) (18). NTg, nontransgenic; Tglo, low expressor; Tghi, high expressor. (B) Assocation of endogenous IκBα (E) and transgene-encoded IκBα (ΔN) with the RelA subunit of NF-κB. Isolated thymocytes from the indicated sources were cultured for 0.5 h in the presence of cycloheximide (50 μg/ml) to arrest translation and then treated with PMA and ionomycin for 0.5 h as indicated. Proteins were immunoprecipitated from cytosolic extracts using a RelA-specific antiserum and subjected to immunoblot analysis as in A. Similar results were obtained after immunoprecipitation with c-Rel–specific antibodies (data not shown). (C) Gel mobility shift analysis of nuclear NF-κB/Rel proteins. Thymocyte suspensions were prepared from the indicated sources and cultured for 0.5 h in the presence or absence of PMA/ionomycin. Equal amounts of nuclear extracts were added to DNA binding mixtures containing a 32P-labeled κB probe (κB-pd) (24). DNA–protein complexes were resolved on nondenaturing polyacrylamide gels and visualized by autoradiography. (D) DNA–protein crosslinking assay of nuclear NF-κB/Rel proteins. DNA binding reaction mixtures (as in C) were irradiated with UV light, fractionated by SDS-PAGE, and visualized by autoradiography (lanes 1–6) (18, 24, 25). Alternatively, portions of reaction mixtures generated for lane 2 were subjected to immunoprecipitation using the indicated antiserum before gel electrophoresis (lanes 7–9). Subunit identities are indicated. (E) Gel mobility shift analysis of nuclear NF-κB/Rel proteins in splenic T cells. B cell–depleted T cell populations (<10% B220+; >80% CD3+) were prepared from splenocytes pooled from three NTg and four Tghi mice. Cells were cultured for 1 h in the presence or absence of PMA/ionomycin (lanes 5–8) in parallel with resting and stimulated thymocytes pooled from the same mice (lanes 1–4). Equal amounts of nuclear extracts were added to DNA binding mixtures containing a 32P-labeled κB probe (κB-pd) (24). DNA–protein complexes were resolved on nondenaturing polyacrylamide gels and visualized by autoradiography. The major inducible complexes (p50–RelA and p50–c-Rel) are indicated with an arrow; the constitutive, higher mobility complexes consist of p50 homodimers (30).
Figure 1
Abrogated expression of NF-κB/Rel in IκBα(ΔN) transgenic mice. (A) Transgeneencoded IκBα expressed in thymocytes and splenocytes. Cytoplasmic extracts from unfractionated cell suspensions were subjected to immunopurification using immobilized anti-FLAG M2 antibodies and resolved by SDSPAGE. Resolved proteins were transferred to polyvinylidene difluoride membranes and probed with an antiserum directed against human IκBα (amino acids 289–317) in conjunction with an enhanced chemiluminescence detection system (Amersham Corp., Arlington Heights, IL) (18). NTg, nontransgenic; Tglo, low expressor; Tghi, high expressor. (B) Assocation of endogenous IκBα (E) and transgene-encoded IκBα (ΔN) with the RelA subunit of NF-κB. Isolated thymocytes from the indicated sources were cultured for 0.5 h in the presence of cycloheximide (50 μg/ml) to arrest translation and then treated with PMA and ionomycin for 0.5 h as indicated. Proteins were immunoprecipitated from cytosolic extracts using a RelA-specific antiserum and subjected to immunoblot analysis as in A. Similar results were obtained after immunoprecipitation with c-Rel–specific antibodies (data not shown). (C) Gel mobility shift analysis of nuclear NF-κB/Rel proteins. Thymocyte suspensions were prepared from the indicated sources and cultured for 0.5 h in the presence or absence of PMA/ionomycin. Equal amounts of nuclear extracts were added to DNA binding mixtures containing a 32P-labeled κB probe (κB-pd) (24). DNA–protein complexes were resolved on nondenaturing polyacrylamide gels and visualized by autoradiography. (D) DNA–protein crosslinking assay of nuclear NF-κB/Rel proteins. DNA binding reaction mixtures (as in C) were irradiated with UV light, fractionated by SDS-PAGE, and visualized by autoradiography (lanes 1–6) (18, 24, 25). Alternatively, portions of reaction mixtures generated for lane 2 were subjected to immunoprecipitation using the indicated antiserum before gel electrophoresis (lanes 7–9). Subunit identities are indicated. (E) Gel mobility shift analysis of nuclear NF-κB/Rel proteins in splenic T cells. B cell–depleted T cell populations (<10% B220+; >80% CD3+) were prepared from splenocytes pooled from three NTg and four Tghi mice. Cells were cultured for 1 h in the presence or absence of PMA/ionomycin (lanes 5–8) in parallel with resting and stimulated thymocytes pooled from the same mice (lanes 1–4). Equal amounts of nuclear extracts were added to DNA binding mixtures containing a 32P-labeled κB probe (κB-pd) (24). DNA–protein complexes were resolved on nondenaturing polyacrylamide gels and visualized by autoradiography. The major inducible complexes (p50–RelA and p50–c-Rel) are indicated with an arrow; the constitutive, higher mobility complexes consist of p50 homodimers (30).
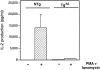
Figure 2
IL-2 production by thymocytes from IκBα(ΔN) transgenic mice. Thymocytes from either NTg or Tghi mice were cultured in triplicate for 48 h in media alone (open bars) or treated with combinations of PMA (50 ng/ml) and ionomycin (1 μg/ml) (stippled bars). Supernatants from these cultures and IL-2 standards were assayed by ELISA. IL-2 production by unstimulated NTg thymocytes was below the detection limit of the assay (3–5 pg IL-2/ml). Results represent the mean values (± SEM) from five separate Tghi mice and five NTg littermates, as analyzed in three separate experiments.
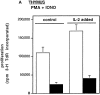
Figure 3
Failure of IL-2 to rescue the proliferative defect in IκBα(ΔN) mice. Thymocytes from either NTg (open bars) or Tghi (closed bars) mice were treated for 40 h with (A) combinations of PMA (50 ng/ml) and ionomycin (1 μg/ml). (B) Con A (2.5 μg/ml), or (C) plate-bound anti-CD3 (10 μg/ml), in the presence or absence of IL-2 (100 U/ml) as indicated. Cells were pulsed for an additional 8 h with tritiated thymidine and harvested for scintillation counting. The results are shown as the mean of tritiated thymidine incorporation (± SEM) for eight Tghi mice and eight NTg littermates (four independent experiments). (D) Splenocytes depleted of B cells and macrophages were analyzed under similar conditions using plate-bound anti-CD3 (10 μg/ml), agonistic antibodies against CD28 (10 μg/ml), and IL-2 (100 U/ml) as indicated.
Figure 3
Failure of IL-2 to rescue the proliferative defect in IκBα(ΔN) mice. Thymocytes from either NTg (open bars) or Tghi (closed bars) mice were treated for 40 h with (A) combinations of PMA (50 ng/ml) and ionomycin (1 μg/ml). (B) Con A (2.5 μg/ml), or (C) plate-bound anti-CD3 (10 μg/ml), in the presence or absence of IL-2 (100 U/ml) as indicated. Cells were pulsed for an additional 8 h with tritiated thymidine and harvested for scintillation counting. The results are shown as the mean of tritiated thymidine incorporation (± SEM) for eight Tghi mice and eight NTg littermates (four independent experiments). (D) Splenocytes depleted of B cells and macrophages were analyzed under similar conditions using plate-bound anti-CD3 (10 μg/ml), agonistic antibodies against CD28 (10 μg/ml), and IL-2 (100 U/ml) as indicated.
Figure 3
Failure of IL-2 to rescue the proliferative defect in IκBα(ΔN) mice. Thymocytes from either NTg (open bars) or Tghi (closed bars) mice were treated for 40 h with (A) combinations of PMA (50 ng/ml) and ionomycin (1 μg/ml). (B) Con A (2.5 μg/ml), or (C) plate-bound anti-CD3 (10 μg/ml), in the presence or absence of IL-2 (100 U/ml) as indicated. Cells were pulsed for an additional 8 h with tritiated thymidine and harvested for scintillation counting. The results are shown as the mean of tritiated thymidine incorporation (± SEM) for eight Tghi mice and eight NTg littermates (four independent experiments). (D) Splenocytes depleted of B cells and macrophages were analyzed under similar conditions using plate-bound anti-CD3 (10 μg/ml), agonistic antibodies against CD28 (10 μg/ml), and IL-2 (100 U/ml) as indicated.
Figure 3
Failure of IL-2 to rescue the proliferative defect in IκBα(ΔN) mice. Thymocytes from either NTg (open bars) or Tghi (closed bars) mice were treated for 40 h with (A) combinations of PMA (50 ng/ml) and ionomycin (1 μg/ml). (B) Con A (2.5 μg/ml), or (C) plate-bound anti-CD3 (10 μg/ml), in the presence or absence of IL-2 (100 U/ml) as indicated. Cells were pulsed for an additional 8 h with tritiated thymidine and harvested for scintillation counting. The results are shown as the mean of tritiated thymidine incorporation (± SEM) for eight Tghi mice and eight NTg littermates (four independent experiments). (D) Splenocytes depleted of B cells and macrophages were analyzed under similar conditions using plate-bound anti-CD3 (10 μg/ml), agonistic antibodies against CD28 (10 μg/ml), and IL-2 (100 U/ml) as indicated.
Figure 4
Increased apoptosis among activated T cells from IκBα(ΔN) transgenic mice. (A) Splenocytes from either NTg (open bars) or Tghi (closed bars) mice were cultured in the presence of Con A (2.5 μg/ml). After 40 h in culture, cells were divided equally, stained with PE-labeled antibodies against CD4 or CD8, and subjected to TUNEL analysis. The data represent the mean percentage (± SEM) of TUNEL-positive cells in each T cell subset. The mean was calculated using data from 19 individual Tghi mice or an equal number of NTg controls (10 independent experiments with 6–8-wk-old mice). The increased frequencies of apoptotic cells among transgenic CD4+ and CD8+ cells were statistically significant when compared with nontransgenic CD4+ or CD8+ cells (P <0.001). (B) Splenocytes were activated using plate-bound anti-CD3, then processed for TUNEL assays as in A. Results represent cumulative data from 12 individual Tghi mice or an equal number of NTg controls (six independent experiments with 6-8-wk-old mice). The increased frequencies of apoptotic cells among transgenic CD4+ and CD8+ cells were statistically significant when compared with nontransgenic CD4+ or CD8+ cells (P ⩽0.001).
Figure 5
Decreased thymic CD8+ cells in IκBα(ΔN) transgenic mice. (A) A representative FACS® profile from three-color indirect immunofluorescence experiments. Thymocytes from NTg and Tghi mice were analyzed by flow cytometry for surface expression of CD4 and CD8 on cells bearing high density TCR-α/β (TCR high) or medium to low density receptors (TCR med/low). Gating of cells based on TCR-α/β expression is shown to the left, together with the percentage of total cells in each gate. Dual parameter fluorescence histograms (CD4 versus CD8) derived from the gated subsets (TCRmed/lo and TCRhi, respectively) are shown on the right. Numbers represent the percentage of gated thymocytes in each quadrant. Mean cell counts were equivalent in NTg and Tg hi thymuses (99 [± 9.1] × 106 versus 97.7 [± 10.1] × 106 respectively). (B) Subset distribution of the TCRhi thymocyte populations in NTg (open bars) and Tg hi (closed bars) mice. Mean cell populations (± SEM) or TCRhi cells in the CD4+ CD8− and CD4− CD8+ quadrants are shown (n = 10). There was no significant difference in mean numbers of CD4− CD8− or CD4+ CD8+ cells.
Figure 5
Decreased thymic CD8+ cells in IκBα(ΔN) transgenic mice. (A) A representative FACS® profile from three-color indirect immunofluorescence experiments. Thymocytes from NTg and Tghi mice were analyzed by flow cytometry for surface expression of CD4 and CD8 on cells bearing high density TCR-α/β (TCR high) or medium to low density receptors (TCR med/low). Gating of cells based on TCR-α/β expression is shown to the left, together with the percentage of total cells in each gate. Dual parameter fluorescence histograms (CD4 versus CD8) derived from the gated subsets (TCRmed/lo and TCRhi, respectively) are shown on the right. Numbers represent the percentage of gated thymocytes in each quadrant. Mean cell counts were equivalent in NTg and Tg hi thymuses (99 [± 9.1] × 106 versus 97.7 [± 10.1] × 106 respectively). (B) Subset distribution of the TCRhi thymocyte populations in NTg (open bars) and Tg hi (closed bars) mice. Mean cell populations (± SEM) or TCRhi cells in the CD4+ CD8− and CD4− CD8+ quadrants are shown (n = 10). There was no significant difference in mean numbers of CD4− CD8− or CD4+ CD8+ cells.
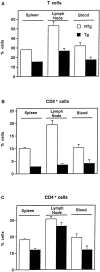
Figure 6
Altered T cell subsets in IκBα(ΔN) transgenic mice. Cells from the indicated peripheral sites were stained with fluorochrome-conjugated mAbs against CD3 (A), CD8 (B), or CD4 (C) and analyzed by flow cytometry. Mean cell counts were equivalent in NTg and Tg hi spleens (77.9 [± 7.2] versus 72.7 [± 5.0] × 106 cells, respectively; P >0.5), and reduced in Tghi lymph nodes (9.25 [± 1.6] × 106 cells) relative to NTg samples (15.0 [± 2.6] × 106 cells; P = 0.09). Therefore, the data are presented as the percentage of cells (± SEM) expressing these surface markers in spleen (n = 22), lymph node (n = 11), or blood (n = 9). Differences between NTg (open bars) and Tghi (closed bars) samples were significant at P <0.0001 (spleen) and P <0.001 (blood, lymph node) with the exception of CD4+ lymph node cells. Analysis of T cells at each of these sites failed to reveal the CD8lo population previously linked to superantigen-induced apoptosis and CD8+ T cell loss (26). Evidence of T cell hyperactivation (50) was absent. Splenic cellularity was normal in two independent Tglo lines (n = 9). CD4+ and CD8+ cells represented 17.5 ± 1.6% and 5.0 ± 0.8% of Tglo splenocytes (CD4/CD8 ratio = 4.6 ± 0.9). In contrast, CD4+ and CD8+ cells represented 20.1 ± 1.0% and 10.0 ± 0.55% of splenocytes from littermate controls. The observed differences in CD8+ cell numbers and CD4/CD8 ratios were significant at P <0.001 and P <0.05, respectively.
Similar articles
- Inefficient ZAP-70 phosphorylation and decreased thymic selection in vivo result from inhibition of NF-kappaB/Rel.
Mora AL, Stanley S, Armistead W, Chan AC, Boothby M. Mora AL, et al. J Immunol. 2001 Nov 15;167(10):5628-35. doi: 10.4049/jimmunol.167.10.5628. J Immunol. 2001. PMID: 11698434 - Costimulation reverses the defect in IL-2 but not effector cytokine production by T cells with impaired IkappaBalpha degradation.
Aune TM, Mora AL, Kim S, Boothby M, Lichtman AH. Aune TM, et al. J Immunol. 1999 May 15;162(10):5805-12. J Immunol. 1999. PMID: 10229814 - Mechanism responsible for T-cell antigen receptor- and CD28- or interleukin 1 (IL-1) receptor-initiated regulation of IL-2 gene expression by NF-kappaB.
Kalli K, Huntoon C, Bell M, McKean DJ. Kalli K, et al. Mol Cell Biol. 1998 Jun;18(6):3140-8. doi: 10.1128/MCB.18.6.3140. Mol Cell Biol. 1998. PMID: 9584155 Free PMC article. - Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo.
Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Jones RG, et al. J Exp Med. 2000 May 15;191(10):1721-34. doi: 10.1084/jem.191.10.1721. J Exp Med. 2000. PMID: 10811865 Free PMC article.
Cited by
- NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer.
Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. Kisseleva T, et al. J Clin Invest. 2006 Nov;116(11):2955-63. doi: 10.1172/JCI27392. Epub 2006 Oct 19. J Clin Invest. 2006. PMID: 17053836 Free PMC article. - Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8(+) but not CD4(+) T cells.
Merritt C, Enslen H, Diehl N, Conze D, Davis RJ, Rincón M. Merritt C, et al. Mol Cell Biol. 2000 Feb;20(3):936-46. doi: 10.1128/MCB.20.3.936-946.2000. Mol Cell Biol. 2000. PMID: 10629051 Free PMC article. - Stimulus-dependent synergism of the antiapoptotic tumor necrosis factor receptor-associated factor 2 (TRAF2) and nuclear factor kappaB pathways.
Lee SY, Kaufman DR, Mora AL, Santana A, Boothby M, Choi Y. Lee SY, et al. J Exp Med. 1998 Oct 5;188(7):1381-4. doi: 10.1084/jem.188.7.1381. J Exp Med. 1998. PMID: 9763618 Free PMC article. - Differential role of the transcription factor NF-kappaB in selection and survival of CD4+ and CD8+ thymocytes.
Jimi E, Strickland I, Voll RE, Long M, Ghosh S. Jimi E, et al. Immunity. 2008 Oct 17;29(4):523-37. doi: 10.1016/j.immuni.2008.08.010. Immunity. 2008. PMID: 18957265 Free PMC article. - An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis.
Hettmann T, DiDonato J, Karin M, Leiden JM. Hettmann T, et al. J Exp Med. 1999 Jan 4;189(1):145-58. doi: 10.1084/jem.189.1.145. J Exp Med. 1999. PMID: 9874571 Free PMC article.
References
- Jamieson C, McCaffrey PG, Rao A, Sen R. Physiologic activation of T cells via the T cell receptor induces NF-kappa B. J Immunol. 1991;147:416–420. - PubMed
- Kang S-M, Tran A-C, Grilli M, Lenardo MJ. NF-κB subunit regulation in non-transformed CD4+ T lymphocytes. Science (Wash DC) 1992;256:1452–1456. - PubMed
- Sen J, Venkataraman L, Shinkai Y, Pierce JW, Alt FW, Burakoff SJ, Sen R. Expression and induction of nuclear factor-κB–related proteins in thymocytes. J Immunol. 1995;154:3213–3221. - PubMed
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials