Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration - PubMed (original) (raw)
Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration
P F Mühlradt et al. J Exp Med. 1997.
Abstract
Macrophages are typically stimulated by components of microbial cell walls. Surprisingly, cell wall-less mycoplasmas can also very efficiently stimulate macrophages. We showed recently that mycoplasma-derived lipopeptides constitute the active principle. We have now isolated a clone of Mycoplasma fermentans expressing mainly one macrophage-stimulating lipopeptide. This lipopeptide was detergent-extracted and isolated by reversed-phase high-performance liquid chromotography, using nitric oxide release from C3H/HeJ mouse macrophages as bioassay for detection. In contrast to "conventional" bacterial lipoproteins, this lipopeptide had a free NH2 terminus. Amino acid composition, sequence, and the molecular weight of 2,163. 3 are consistent with the following structure: S-(2, 3-bisacyloxypropyl)cysteine-GNNDESNISFKEK with one mole C16:0, and a further mole of a mixture of C18:0 and C18:1 fatty acid per lipopeptide molecule. The sequence could not be found in either the protein identification resource nor the Swiss Prot data bank. We named this 2-kD lipopeptide, macrophage-activating lipopeptide-2 (MALP-2). Synthetic dipalmitoyl MALP-2 and mycoplasma-derived MALP-2 were compared with the bioassay. Both lipopeptides showed an identical dose dependency with a half-maximal response at 10(-11) M concentration. MALP-2 may be one of the most potent natural macrophage stimulators besides endotoxin.
Figures
Figure 1
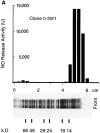
Macrophage-stimulatory activity of clones from M. fermentans. Individual colonies grown on agar were picked and then cultured in liquid medium. Mycoplasmas were harvested and MSA was extracted with hot octyl glucoside and determined in the NO release assay with IFN-γ–treated peritoneal exudate cells from LPS low-responder mice. MSA was calculated as U/mg mycoplasma protein.
Figure 2
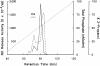
Comparison of silver stain and MSA in gel slices after SDS-PAGE of M. fermentans PG 18 type strain and clone II-29/1. Samples were run in 15% gels in the discontinuous buffer system of Lämmli under reducing conditions, applying 10 μg mycoplasma protein per lane for silver staining. 70 μg were subjected to electrophoresis on a neighboring lane. This lane was cut in 3-mm segments which were extracted in 0.3 ml hot octyl glucoside for subsequent determination of MSA.
Figure 2
Comparison of silver stain and MSA in gel slices after SDS-PAGE of M. fermentans PG 18 type strain and clone II-29/1. Samples were run in 15% gels in the discontinuous buffer system of Lämmli under reducing conditions, applying 10 μg mycoplasma protein per lane for silver staining. 70 μg were subjected to electrophoresis on a neighboring lane. This lane was cut in 3-mm segments which were extracted in 0.3 ml hot octyl glucoside for subsequent determination of MSA.
Figure 3
HPLC of octyl glucoside extracted MSA from M. fermentans. MSA was extracted as in Table 1. 2.6 × 107 U were applied to a 10 × 250–mm RP8 reversed phase column and eluted with 2-propanol. (solid line) MSA as determined in the NO release assay; (dotted line) inorganic phosphate to monitor phospholipids. The bar below OG shows where octyl glucoside elutes from the column.
Figure 4
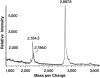
MALDI spectrum of HPLC-purified MALP-2. The lipopeptide gave rise to a [M + H]+ ion at m/z 2164.3 and a [M + Na]+ ion at m/z 2186.0. The signal at m/z 2867.8 is due to the [M + 2H]2+ signal of the internal calibration standard bovine insulin.
Figure 5
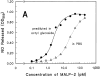
Concentration dependency of MALP-2 in the NO release assay. (A) HPLC-purified MALP-2 from M. fermentans clone II-29/1 prediluted in octyl glucoside (solid line) or PBS (dotted line). (B) HPLC-purified MALP-2 (solid line) and synthetic dipalmitoyl MALP-2 (dotted line), both prediluted in octyl glucoside. The diamond on the y-axis shows NO release of control cells with IFN-γ only. Data are the average of triplicate determinations ± standard deviation.
Figure 5
Concentration dependency of MALP-2 in the NO release assay. (A) HPLC-purified MALP-2 from M. fermentans clone II-29/1 prediluted in octyl glucoside (solid line) or PBS (dotted line). (B) HPLC-purified MALP-2 (solid line) and synthetic dipalmitoyl MALP-2 (dotted line), both prediluted in octyl glucoside. The diamond on the y-axis shows NO release of control cells with IFN-γ only. Data are the average of triplicate determinations ± standard deviation.
Similar articles
- Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis.
Mühlradt PF, Kiess M, Meyer H, Süssmuth R, Jung G. Mühlradt PF, et al. Infect Immun. 1998 Oct;66(10):4804-10. doi: 10.1128/IAI.66.10.4804-4810.1998. Infect Immun. 1998. PMID: 9746582 Free PMC article. - Identification of S-(2,3-dihydroxypropyl)cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans.
Mühlradt PF, Meyer H, Jansen R. Mühlradt PF, et al. Biochemistry. 1996 Jun 18;35(24):7781-6. doi: 10.1021/bi9602831. Biochemistry. 1996. PMID: 8672478 - Pushing the envelope: Immune mechanism and application landscape of macrophage-activating lipopeptide-2.
Liao D, Su X, Wang J, Yu J, Luo H, Tian W, Ye Z, He J. Liao D, et al. Front Immunol. 2023 Jan 24;14:1113715. doi: 10.3389/fimmu.2023.1113715. eCollection 2023. Front Immunol. 2023. PMID: 36761746 Free PMC article. Review. - A lipoprotein family from Mycoplasma fermentans confers host immune activation through Toll-like receptor 2.
Seya T, Matsumoto M. Seya T, et al. Int J Biochem Cell Biol. 2002 Aug;34(8):901-6. doi: 10.1016/s1357-2725(01)00164-9. Int J Biochem Cell Biol. 2002. PMID: 12007626 Review.
Cited by
- Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6.
Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, Kuroki Y, Ogawa T, Shibata K. Okusawa T, et al. Infect Immun. 2004 Mar;72(3):1657-65. doi: 10.1128/IAI.72.3.1657-1665.2004. Infect Immun. 2004. PMID: 14977973 Free PMC article. - Identification and functional mapping of the Mycoplasma fermentans P29 adhesin.
Leigh SA, Wise KS. Leigh SA, et al. Infect Immun. 2002 Sep;70(9):4925-35. doi: 10.1128/IAI.70.9.4925-4935.2002. Infect Immun. 2002. PMID: 12183538 Free PMC article. - Incorporation of a Toll-like receptor 2/6 agonist potentiates mRNA vaccines against cancer and infectious diseases.
Gu Y, Yang J, He C, Zhao T, Lu R, Liu J, Mo X, Wen F, Shi H. Gu Y, et al. Signal Transduct Target Ther. 2023 Jul 17;8(1):273. doi: 10.1038/s41392-023-01479-4. Signal Transduct Target Ther. 2023. PMID: 37455272 Free PMC article. - Phase variations of the Mycoplasma penetrans main surface lipoprotein increase antigenic diversity.
Neyrolles O, Chambaud I, Ferris S, Prevost MC, Sasaki T, Montagnier L, Blanchard A. Neyrolles O, et al. Infect Immun. 1999 Apr;67(4):1569-78. doi: 10.1128/IAI.67.4.1569-1578.1999. Infect Immun. 1999. PMID: 10084988 Free PMC article. - Mono-Palmitoyl-N-Alkylurea Ligands as Specific Activators of Human Toll-Like Receptor 2/6 Heterodimer.
Isendoorn MME, Castello G, Koç Ç, Meeuwenoord N, Codée JDC, Ossendorp F, Filippov DV. Isendoorn MME, et al. Chembiochem. 2024 Dec 2;25(23):e202400583. doi: 10.1002/cbic.202400583. Epub 2024 Nov 11. Chembiochem. 2024. PMID: 39381901 Free PMC article.
References
- Ruuth E, Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989;112:133–160. - PubMed
- Yang G, Coffman FD, Wheelock EF. Characterization and purification of a macrophage-triggering factor produced in Mycoplasma arginini-infected L5178Y cell cultures. J Immunol. 1994;153:2579–2591. - PubMed
- Horner PJ, Gilroy CB, Thomas BJ, Naidoo ROM, Taylor-Robinson D. Association of Mycoplasma genitaliumwith acute non-gonococcal urethritis. Lancet. 1993;342:582–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources