Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons - PubMed (original) (raw)
Comparative Study
Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons
I M Raman et al. J Neurosci. 1997.
Abstract
Voltage-dependent sodium channels were studied in dissociated cerebellar Purkinje neurons from rats. In whole-cell recordings, a tetrodotoxin (TTX)-sensitive inward current was elicited when the membrane was repolarized to voltages between -60 and -20 mV after depolarizations to +30 mV long enough to produce maximal inactivation. At -40 mV, this "resurgent" current peaked in 8 msec and decayed with a time constant of 30 msec. With 50 mM sodium as a charge carrier, the resurgent current was on average approximately 120 pA. CA3 pyramidal neurons had no such current. The current may reflect recovery of inactivated channels through open states, because in Purkinje neurons (but not CA3 neurons) there was partial recovery from inactivation at -40 mV, coinciding with the rise of resurgent current. In single-channel recordings, individual channels gave openings corresponding to resurgent and conventional transient current. Action potentials were recorded from dissociated neurons under current clamp to investigate the role of the resurgent current in action potential formation. Purkinje neurons fired spontaneously at approximately 30 Hz. Hyperpolarization to -85 mV prevented spontaneous firing, and brief depolarization then induced all-or-none firing of conglomerate action potentials comprising three to four spikes. When conglomerate action potentials were used as command voltages in voltage-clamp experiments, TTX-sensitive sodium current was elicited between spikes. The falling phase of an action potential is similar to voltage patterns that activate resurgent sodium current, and thus, resurgent sodium current likely contributes to the formation of conglomerate action potentials in Purkinje neurons.
Figures
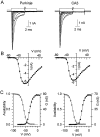
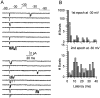
Fig. 1.
Transient sodium currents in Purkinje and CA3 neurons. A, Currents evoked from a holding potential of −90 mV by 50 msec steps to potentials between −80 and +50 mV in 10 mV increments are shown for a Purkinje cell (left) and a CA3 cell (right). The break in the trace represents 39 msec. B, Current–voltage relation for the peak currents in A. The points are connected by a spline function for clarity. C, Peak conductance (filled circles) and steady-state inactivation curves (open circles) for the cells shown in A. Conductance curves were fitted (lines) with the Boltzmann function,G = _G_max/(1 + exp[−(V −_V_h)/_k_]), where G is conductance in nanosiemens, _G_max is the maximal conductance, V is the step potential in mV,_V_h is the voltage for half-maximal activation in mV, and k is the slope factor in mV. The fitted _G_max, _V_h, and k for the Purkinje neuron were 76.4 nS, −32.3 mV, and 5.5 mV, and for the CA3 neuron 66.7 nS, −28.4 mV, and 4.8 mV. The inactivation curves were fitted by the Boltzmann function 1/(1 + exp(V −_V_h)/k)._V_h and k values were −56.5 and 5.8 mV for the Purkinje cell (left), and −67 and 6.2 mV for the CA3 cell (right).
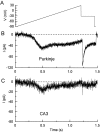
Fig. 2.
Sodium currents elicited by slow ramps in Purkinje and CA3 neurons. The membrane voltage was ramped from −90 mV to +30 mV at 0.1 mV/msec (A). Currents evoked by this protocol were recorded in a Purkinje neuron (B) and a CA3 neuron (C). In both cases, sodium current was determined by subtracting control current from that in 300 n
m
TTX (which seemed to leave only leak and capacity current). In both the Purkinje neuron and CA3 neuron, the maximal current during the ramp occurred at −40 mV. The peak transient current at −30 mV in the CA3 cell was 66% of that in the Purkinje cell, hence the scaling of the ordinates.
Fig. 3.
Resurgent sodium current. A, Sodium current was evoked by a 20 msec step from −90 to +30 mV, after which the membrane was repolarized to voltages between −20 and −60 mV.B, TTX-sensitive sodium current elicited by this protocol in a Purkinje neuron. The transient current at +30 mV is offscale (peak of 2 nA). Leak and capacity currents in 300 n
m
TTX were subtracted. C, TTX-sensitive sodium current elicited by the same protocol in a CA3 neuron. Peak current at +30 mV was 1.3 nA (offscale). Scale bars apply to both sets of traces.
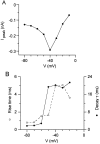
Fig. 4.
Voltage dependence of resurgent sodium current. A, Peak resurgent sodium current (after 20 msec step to +30 mV) versus repolarization potential for the Purkinje cell in Figure 3. At −10 mV, there was no peak current, but the steady-state current of −50 pA is plotted. B, Rise time (time to peak) and decay time constants for the resurgent sodium currents.
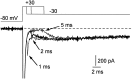
Fig. 5.
Resurgent sodium current after brief steps to +30 mV. Resurgent current at −30 mV is shown after steps to +30 mV of 1, 2, and 5 msec. After the briefest steps to +30 mV, tail currents (arrows) are followed by the resurgent current. The peak current at +30 mV (offscale) is −3100 pA.
Fig. 6.
Single sodium channels in Purkinje neurons.A, Two sets of eight consecutive traces recorded from a one-channel patch are shown. Fourteen hundred such sweeps were recorded from this patch. B, Latency histograms of bursts during the first and second epochs at −30 mV.
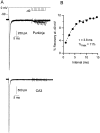
Fig. 7.
Recovery from inactivation at −40 mV in Purkinje and CA3 neurons. A, Responses to the voltage protocol shown for a Purkinje cell (top traces) and CA3 cell (bottom traces). The initial conditioning step from −90 to 0 mV was 25 msec. Intervals at −40 mV were 1–14.5 msec in 1.5 msec increments. B, Amplitudes of recovery currents were normalized to the peak current in response to the conditioning step, and the percentage of recovery is plotted against interval. Data were fitted with a single exponential function,%recovery=A*exp(−t/τ)+%max,where %max is the maximal recovery, _t_is the interval (msec), τ is the time constant of recovery, and %max + A is the noninactivated percentage of current (0.77% for this cell).
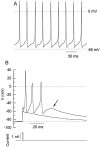
Fig. 8.
Spontaneous firing and conglomerate action potentials in acutely isolated Purkinje cells. A, Spontaneous action potentials recorded in an isolated Purkinje cell.B, Voltage responses of a different Purkinje cell in response to a 1 msec current injection of 1.2 and 1.4 nA. The smaller injection produced the subthreshold response. A steady injection of −40 pA brought the resting potential to −85 mV and stopped the spontaneous firing. Note different time scale from_A_.
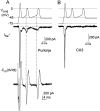
Fig. 9.
Sodium currents evoked by conglomerate action potential waveforms in Purkinje and CA3 neurons.A, The top panel illustrates the command potential (_V_cmd) applied to a voltage-clamped Purkinje cell. The command waveform was obtained from a conglomerate action potential. _Thin horizontal lines_indicate 0, −45, and −75 mV, on _V_cmd as labeled. The middle panel (heavy line) shows the Na+ current (in 50 m
m
Na+) evoked in this cell by the voltage protocol. The_bottom panel_ shows the total current flux in physiological solution (140 Na+ plus K+ and Ca2+; see Materials and Methods) calculated from the product of −dV/dt of the spike waveform and the cell capacitance _C_m of the cell in which the conglomerate action potential was recorded. The total current is plotted on the same scale as the_I_Na+ to facilitate comparison. The peak_I_total is −3525 pA and is offscale in the figure. Dashed lines at 0 pA are included on all the current traces. Vertical dotted lines indicate the relative amplitudes of I_Na+ and_I_total at the onset of the second and third action potentials. B, Same voltage protocol as_A applied to a CA3 cell.
Fig. 10.
Inactivation and recovery of Na+current during the conglomerate action potential. A, Responses of a Purkinje cell evoked by the voltage protocol shown. After increasing durations of the action potential waveform, test current was elicited at 0 mV (after a 0.5-msec step to −100 mV). Durations of the action potential waveform (and voltages reached) were 2.85 msec (10 mV), 3.2 msec (−10 mV, bold traces), 3.65 msec (−30 mV), 4.95 msec (−46 mV), 6.35 msec (−43 mV), and 7.35 msec (−39 mV). The inset to A plots the percentage of recovery on the same time base as the traces, for the Purkinje cell shown (filled symbols), and for a representative CA3 cell (open symbols). Percentage of recovery was calculated as the peak test current at 0 mV normalized to the maximal current evoked by a step to 0 directly from the −75 mV holding potential. The amount of recovery that occurs during a half msec at −100 mV, ∼7%, is reflected by the dotted line (see traces in B).B, The response of the Purkinje cell in _A_to the step protocol shown.
Similar articles
- Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin.
Grieco TM, Raman IM. Grieco TM, et al. J Neurosci. 2004 Jan 7;24(1):35-42. doi: 10.1523/JNEUROSCI.3807-03.2004. J Neurosci. 2004. PMID: 14715935 Free PMC article. - The absence of resurgent sodium current in mouse spinal neurons.
Pan F, Beam KG. Pan F, et al. Brain Res. 1999 Dec 4;849(1-2):162-8. doi: 10.1016/s0006-8993(99)02060-0. Brain Res. 1999. PMID: 10592298 - The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study.
Khaliq ZM, Gouwens NW, Raman IM. Khaliq ZM, et al. J Neurosci. 2003 Jun 15;23(12):4899-912. doi: 10.1523/JNEUROSCI.23-12-04899.2003. J Neurosci. 2003. PMID: 12832512 Free PMC article. - Resurgent current of voltage-gated Na(+) channels.
Lewis AH, Raman IM. Lewis AH, et al. J Physiol. 2014 Nov 15;592(22):4825-38. doi: 10.1113/jphysiol.2014.277582. Epub 2014 Aug 28. J Physiol. 2014. PMID: 25172941 Free PMC article. Review. - Voltage-gated sodium currents in cerebellar Purkinje neurons: functional and molecular diversity.
Ransdell JL, Nerbonne JM. Ransdell JL, et al. Cell Mol Life Sci. 2018 Oct;75(19):3495-3505. doi: 10.1007/s00018-018-2868-y. Epub 2018 Jul 7. Cell Mol Life Sci. 2018. PMID: 29982847 Free PMC article. Review.
Cited by
- Aberrant Purkinje cell activity is the cause of dystonia in a shRNA-based mouse model of Rapid Onset Dystonia-Parkinsonism.
Fremont R, Tewari A, Khodakhah K. Fremont R, et al. Neurobiol Dis. 2015 Oct;82:200-212. doi: 10.1016/j.nbd.2015.06.004. Epub 2015 Jun 17. Neurobiol Dis. 2015. PMID: 26093171 Free PMC article. - A model of on/off transitions in neurons of the deep cerebellar nuclei: deciphering the underlying ionic mechanisms.
Berry H, Genet S. Berry H, et al. J Math Neurosci. 2021 Apr 1;11(1):7. doi: 10.1186/s13408-021-00105-3. J Math Neurosci. 2021. PMID: 33796951 Free PMC article. - The Biophysical Basis Underlying Gating Changes in the p.V1316A Mutant Nav1.7 Channel and the Molecular Pathogenesis of Inherited Erythromelalgia.
Huang CW, Lai HJ, Huang PY, Lee MJ, Kuo CC. Huang CW, et al. PLoS Biol. 2016 Sep 21;14(9):e1002561. doi: 10.1371/journal.pbio.1002561. eCollection 2016 Sep. PLoS Biol. 2016. PMID: 27653502 Free PMC article. - Infant and adult SCA13 mutations differentially affect Purkinje cell excitability, maturation, and viability in vivo.
Hsieh JY, Ulrich BN, Issa FA, Lin MA, Brown B, Papazian DM. Hsieh JY, et al. Elife. 2020 Jul 9;9:e57358. doi: 10.7554/eLife.57358. Elife. 2020. PMID: 32644043 Free PMC article. - Cav1.3 Channels as Key Regulators of Neuron-Like Firings and Catecholamine Release in Chromaffin Cells.
Vandael DH, Marcantoni A, Carbone E. Vandael DH, et al. Curr Mol Pharmacol. 2015;8(2):149-61. doi: 10.2174/1874467208666150507105443. Curr Mol Pharmacol. 2015. PMID: 25966692 Free PMC article. Review.
References
- Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969;32:1044–1055. - PubMed
- Brown A, Schwindt P, Crill W. Different voltage dependence of transient and persistent Na+ currents is compatible with modal-gating hypothesis for Na+ channels. J Neurophysiol. 1994;71:2562–2565. - PubMed
- Cardozo DL, Bean BP. Voltage-dependent calcium channels in rat midbrain dopamine neurons: modulation by dopamine and GABAB receptors. J Neurophysiol. 1995;74:1137–1148. - PubMed
- Cepeda C, Chandler SH, Shumate LW, Levine MS. Persistent Na+ conductance in medium-sized neostriatal neurons: characterization using infrared videomicroscopy and whole-cell patch-clamp recordings. J Neurophysiol. 1995;74:1343–1348. - PubMed
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous