Differential susceptibility to neurotoxicity mediated by neurotrophins and neuronal nitric oxide synthase - PubMed (original) (raw)
Comparative Study
Differential susceptibility to neurotoxicity mediated by neurotrophins and neuronal nitric oxide synthase
A F Samdani et al. J Neurosci. 1997.
Abstract
NMDA neurotoxicity, which is mediated, in part, by formation of nitric oxide (NO) via activation of neuronal NO synthase (nNOS), is modulated by neurotrophins. nNOS expression in rat and mouse primary neuronal cultures grown on a glial feeder layer is significantly less than that of neurons grown on a polyornithine (Poly-O) matrix. Neurotrophins markedly increase the number of nNOS neurons, nNOS protein, and NOS catalytic activity and enhance NMDA neurotoxicity via NO-dependent mechanisms when neurons are grown on glial feeder layers. In contrast, when rat or mouse primary cortical neurons are grown on a Poly-O matrix, neurotrophins have no influence on nNOS neuronal number or NOS catalytic activity and reduce NMDA neurotoxicity. Primary neuronal cultures from mice lacking nNOS grown on a glial feeder layer fail to respond to neurotrophin-mediated enhancement of neurotoxicity. Together, these results indicate that nNOS expression and NMDA NO-mediated neurotoxicity are dependent, in part, on the culture paradigm, and neurotrophins regulate the susceptibility to NMDA neurotoxicity via modulation of nNOS. Furthermore, these results support the idea that NMDA neurotoxicity in culture is critically dependent on the developmental state of the neurons being assessed and suggest that, when cortical neurons are cultured on a glial feeder layer, they do not reach nearly as mature a phenotype as when grown on a Poly-O matrix.
Figures
Fig. 1.
Culture-dependent regulation of nNOS expression. Shown are Hoffman modulation photomicrographs of cortical neurons stained with NADPH–diaphorase depicting neuronal nitric oxide synthase (nNOS) neurons 24 hr after treatment with brain-derived neurotrophic factor (BDNF; 100 ng/ml). A,B, Control cultures grown either on a glial layer or a polyornithine (Poly-O Matrix) matrix, respectively.C, D, Cultures that were treated for 24 hr with 100 ng/ml BDNF. Neurons cultured on a glial layer express negligible levels of nNOS neurons, the expression of which is increased markedly with BDNF treatment (A, C). Neurons cultured on a Poly-O matrix have a relatively higher expression of nNOS neurons (arrowheads), which is unchanged with BDNF treatment (B, D).
Fig. 2.
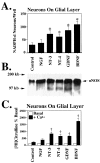
Neurotrophins enhance nNOS expression in cortical neurons grown on a glial layer. A, Pretreatment (24 hr) with neurotrophins (100 ng/ml) markedly enhances the number of nNOS neurons, as indicated by NADPH–diaphorase staining of cortical neurons. Each data point represents the mean ± SEM (n = 8–12) of at least two separate experiments. *p < 0.0001 for NT-3, NT-4, GDNF, and BDNF, as compared with control cultures.NGF is not significantly different from control.B, Neurotrophins increase the amount of_nNOS_ protein, as measured by Western blot analysis.C, A parallel increase in NOS catalytic activity is observed. †p < 0.02 for NT-3, NT-4, GDNF, and BDNF, as compared with control cultures. Each data point represents the mean ± SEM of at least two separate experiments performed in duplicate.
Fig. 3.
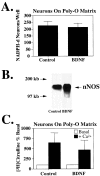
Neurotrophins do not enhance expression of nNOS in cortical neurons grown on a polyornithine (Poly-O) matrix.A, Cortical neurons grown on a Poly-O matrix express a significantly higher quantity of nNOS neurons, as indicated by NADPH–diaphorase staining, than cortical neurons grown on a glial layer. Pretreatment (24 hr) with BDNF does not influence the total number of nNOS neurons. Each data point represents the mean ± SEM (n = 8–12) of at least two separate experiments. B, BDNF increases the amount of nNOS protein, as measured by Western blot analysis; however, (C) nNOS catalytic activity remains unchanged. Each data point represents the mean ± SEM of at least two separate experiments performed in duplicate. The number of NADPH–diaphorase neurons and NOS catalytic activity in BDNF-treated cultures is not significantly different from controls.
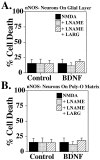
Fig. 4.
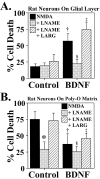
Increases in nNOS levels mediate potentiation of NMDA neurotoxicity by neurotrophins. A, Rat cortical neurons grown on a glial layer exhibit NMDA-mediated (NMDA; 500 μ
m
) neurotoxicity, which is not influenced by the competitive nNOS inhibitor nitroarginine–methyl ester (LNAME; 500 μ
m
) or by excess
l
-arginine (LARG; 5 m
m
). Pretreatment (24 hr) with BDNF (100 ng/ml) markedly enhances NMDA (500 μ
m
) neurotoxicity (†p < 0.0001), which is prevented with LNAME (500 μ
m
;§p < 0.0001), and LARG(5 m
m
; ‡p < 0.0001) reverses this neuroprotection. B, Cortical neurons grown on a Poly-O matrix demonstrate an equivalent amount of_NMDA_ neurotoxicity to that observed in cortical neurons grown on a glial layer and pretreated with BDNF (100 ng/ml). This neurotoxicity is also NO-dependent (*p< 0.0001). BDNF treatment of neurons grown on a Poly-O matrix attenuates the neurotoxic response to NMDA (500 μ
m
) by ∼50% (†p < 0.0001).LNAME (500 μ
m
;§p < 0.05) provides further protection, and LARG (5 m
m
;‡p < 0.0001) reverses this protection. Each data point represents the mean ± SEM (_n_= 6–12) of at least two separate experiments; a minimum of 4000–10,000 neurons was counted.
Fig. 5.
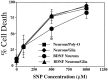
Culture-independent sensitivity to NO neurotoxicity. Cultures were exposed to increasing concentrations of the NO donor sodium nitroprusside (SNP Concentration). Both neuronal culture systems are equally sensitive to the toxic effects of SNP. SNP depleted of NO by preincubating in culture media for 24 hr has no intrinsic toxicity. Pretreatment (24 hr) with_BDNF_ (100 ng/ml) does not influence SNP toxicity in cortical neurons grown on a glial layer; however, cortical neurons grown on a Poly-O matrix are less susceptible to the toxic effect of 500 μ
m
SNP (*p < 0.001). Each data point represents the mean ± SEM (n = 4–6) of at least two separate experiments; a minimum of 4000–8000 neurons was counted.
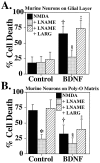
Fig. 6.
Increases in nNOS levels mediate potentiation of NMDA neurotoxicity by neurotrophins. A, Murine cortical neurons grown on a glial layer exhibit NMDA-mediated (NMDA; 500 μ
m
) neurotoxicity, which is not influenced by the competitive nNOS inhibitor nitroarginine–methyl ester (LNAME; 500 μ
m
) or by excess
l
-arginine (LARG; 5 m
m
). Pretreatment (24 hr) with BDNF (100 ng/ml) markedly enhances NMDA (500 μ
m
) neurotoxicity (†p < 0.0001), which is prevented with LNAME (500 μ
m
; §p < 0.0001), and LARG(5 m
m
; ‡p < 0.0001) reverses this neuroprotection. B, Cortical neurons grown on a Poly-O matrix demonstrate an equivalent amount of_NMDA_ (500 μ
m
) NO-dependent neurotoxicity (*p < 0.0001) to that observed in cortical neurons grown on a glial layer pretreated with BDNF (100 ng/ml).BDNF treatment of neurons grown on a Poly-O matrix attenuates the neurotoxic response to NMDA (500 μ
m
) by ∼50% (†p < 0.0001),LNAME (500 μ
m
;§p < 0.01) provides further protection, and LARG (5 m
m
;‡p < 0.0001) reverses this protection. Each data point represents the mean ± SEM (_n_= 6–12) of at least two separate experiments; a minimum of 4000–10,000 neurons was counted.
Fig. 7.
Neurotrophin-mediated enhancement of NMDA neurotoxicity is prevented in nNOS− mice.A, nNOS− cultures are markedly resistant to NMDA neurotoxicity, and BDNF(100 ng/ml) fails to enhance NMDA neurotoxicity in cortical cultures grown on a glial layer. B,BDNF fails to provide neuroprotection to cortical neurons grown on a Poly-O matrix. Each data point represents the mean ± SEM (n = 6–12) of at least two separate experiments; a minimum of 4000–10,000 neurons was counted. None of the determinations is statistically different from another.
Similar articles
- Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice.
Dawson VL, Kizushi VM, Huang PL, Snyder SH, Dawson TM. Dawson VL, et al. J Neurosci. 1996 Apr 15;16(8):2479-87. doi: 10.1523/JNEUROSCI.16-08-02479.1996. J Neurosci. 1996. PMID: 8786424 Free PMC article. - Manganese superoxide dismutase protects nNOS neurons from NMDA and nitric oxide-mediated neurotoxicity.
Gonzalez-Zulueta M, Ensz LM, Mukhina G, Lebovitz RM, Zwacka RM, Engelhardt JF, Oberley LW, Dawson VL, Dawson TM. Gonzalez-Zulueta M, et al. J Neurosci. 1998 Mar 15;18(6):2040-55. doi: 10.1523/JNEUROSCI.18-06-02040.1998. J Neurosci. 1998. PMID: 9482791 Free PMC article. Review. - Targeted disruption of PSD-93 gene reduces platelet-activating factor-induced neurotoxicity in cultured cortical neurons.
Xu Y, Zhang B, Hua Z, Johns RA, Bredt DS, Tao YX. Xu Y, et al. Exp Neurol. 2004 Sep;189(1):16-24. doi: 10.1016/j.expneurol.2004.05.013. Exp Neurol. 2004. PMID: 15296832 - Neuronal NOS: gene structure, mRNA diversity, and functional relevance.
Wang Y, Newton DC, Marsden PA. Wang Y, et al. Crit Rev Neurobiol. 1999;13(1):21-43. doi: 10.1615/critrevneurobiol.v13.i1.20. Crit Rev Neurobiol. 1999. PMID: 10223522 Review.
Cited by
- Nitric oxide signaling participates in norepinephrine-induced activity of neuronal intracellular survival pathways.
Chen MJ, Russo-Neustadt AA. Chen MJ, et al. Life Sci. 2007 Sep 29;81(16):1280-90. doi: 10.1016/j.lfs.2007.09.003. Epub 2007 Sep 15. Life Sci. 2007. PMID: 17915260 Free PMC article. - Inhibition of the p44/42 MAP kinase pathway protects hippocampal neurons in a cell-culture model of seizure activity.
Murray B, Alessandrini A, Cole AJ, Yee AG, Furshpan EJ. Murray B, et al. Proc Natl Acad Sci U S A. 1998 Sep 29;95(20):11975-80. doi: 10.1073/pnas.95.20.11975. Proc Natl Acad Sci U S A. 1998. PMID: 9751775 Free PMC article. - Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase.
Kim SH, Won SJ, Sohn S, Kwon HJ, Lee JY, Park JH, Gwag BJ. Kim SH, et al. J Cell Biol. 2002 Dec 9;159(5):821-31. doi: 10.1083/jcb.200112131. Epub 2002 Dec 2. J Cell Biol. 2002. PMID: 12460985 Free PMC article. - Nitric oxide-stimulated increase in extracellular adenosine accumulation in rat forebrain neurons in culture is associated with ATP hydrolysis and inhibition of adenosine kinase activity.
Rosenberg PA, Li Y, Le M, Zhang Y. Rosenberg PA, et al. J Neurosci. 2000 Aug 15;20(16):6294-301. doi: 10.1523/JNEUROSCI.20-16-06294.2000. J Neurosci. 2000. PMID: 10934281 Free PMC article. - Both the neuronal and inducible isoforms contribute to upregulation of retinal nitric oxide synthase activity by brain-derived neurotrophic factor.
Klöcker N, Kermer P, Gleichmann M, Weller M, Bähr M. Klöcker N, et al. J Neurosci. 1999 Oct 1;19(19):8517-27. doi: 10.1523/JNEUROSCI.19-19-08517.1999. J Neurosci. 1999. PMID: 10493752 Free PMC article.
References
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. - PubMed
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. - PubMed
- Birling MC, Price J. Influence of growth factors on neuronal differentiation. Curr Opin Cell Biol. 1995;7:878–884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources