Lymphotoxin-alpha (LTalpha) supports development of splenic follicular structure that is required for IgG responses - PubMed (original) (raw)
Lymphotoxin-alpha (LTalpha) supports development of splenic follicular structure that is required for IgG responses
Y X Fu et al. J Exp Med. 1997.
Abstract
LTalpha-deficient (LTalpha-/-) mice show altered splenic microarchitecture. This includes loss of normal B cell-T cell compartmentalization, of follicular dendritic cell (FDC) clusters, and of ability to form germinal centers (GC). LTalpha-/- mice immunized with sheep red blood cells (SRBC) produced high levels of antigen-specific IgM but no IgG in either primary or secondary responses, demonstrating failure of Ig class switching. This inability to switch to IgG could have been due to the altered splenic microarchitecture in these mice. Alternatively, it could have been due directly to a requirement for LTalpha expression by lymphocytes cooperating in the antibody response. To investigate this, we performed reciprocal spleen cell transfers. When irradiated LTalpha-/- mice were reconstituted with wild-type splenocytes and immunized immediately with SRBC, splenic microarchitecture remained disturbed and there was no IgG response. In contrast, when irradiated wild-type animals received splenocytes from LTalpha-/- mice, follicle structure and a strong IgG response were retained. These data indicate that LTalpha-deficient B cells and T cells have no intrinsic defect in ability to generate an IgG response. Rather, the altered microenvironment characteristic of LTalpha-/- mice appears to result in impaired ability to switch to a productive IgG response. To investigate whether prolonged expression of LTalpha could alter the structure and function of spleen follicles, reciprocal bone marrow (BM) transplantation was performed. Six weeks after reconstitution of LTalpha-/- mice with wild-type BM, spleen follicle structure was partially restored, with return of FDC clusters and GC. B cell/T cell compartmentalization remained abnormal and white pulp zones were small. This was accompanied by restoration of IgG response to SRBC. Reconstitution of wild-type mice with LTalpha-/- BM resulted in loss of FDC clusters and GC, and loss of the IgG response, although compartmentalized B cell and T cell zones were largely retained. Thus, defective IgG production is not absolutely associated with abnormal B cell and T cell compartmentalization. Rather, expression of LTalpha supports the maturation of spleen follicle structure, including the development and maintenance of FDC clusters, which supports Ig class switching and an effective IgG response.
Figures
Figure 1
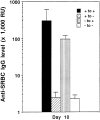
Anti-SRBC IgG responses after subcutaneous or intraperitoneal immunization. Groups of 3–5 mice (8-wk-old) (wild-type, filled symbols; LTα−/−, open symbols) were immunized at day 0 in the footpad (A, 107 SRBC ) or by intraperitoneal injection (B, 108 SRBC) and given a boost with the same dose on day 21. SRBC-specific IgG was measured using an ELISA. Data shown represent the means ± SEM of triplicate determinations from 3–5 mice. RU, relative units. One representative experiment of three is shown.
Figure 2
Anti-SRBC IgM and IgG responses in LTα−/− mice after intravenous immunization. Groups of 3–5 mice (8-wk-old) were immunized i.v. with 108 SRBC in PBS and boosted with the same dose 21 d later. Serum anti-SRBC IgM (A) and IgG (B) were measured by ELISA. Symbols and RU are as described in Fig. 1.
Figure 3
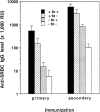
IgG production after spleen cell transfer. Wild-type or LTα−/− mice were irradiated with 750 rad, then treated with an infusion of spleen cells from wild-type or LTα−/− donors together with 108 SRBC. Ten days later, serum was collected and anti-SRBC IgG was measured by ELISA. The results represent mean ± SEM of 3–5 mice per group. This experiment was repeated five times with similar results.
Figure 4
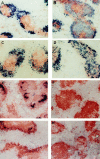
Structure of spleen follicles in irradiated mice reconstituted with wild-type or LTα−/− splenocytes. After serum was collected from mice shown in Fig. 3, the spleens were harvested and frozen sections were stained with anti-B220 (brown) and anti-Thy1.2 (blue) to visualize the B cell and T cell zones (A–D). Distinct B cell and T cell zones were present in wild-type mice that received splenocytes from either normal (A) or LTα−/− mice (C), whereas there was disturbed segregation of B cells and T cells in LTα−/− mice that received splenocytes from either normal (B) or LTα−/− mice (D). FDC clusters were observed by staining with the anti-CR1 monoclonal antibody 8C12 (blue) (E–H). FDC clusters were retained in the spleen follicles of wild-type mice that received splenocytes from either wildtype (E) or LTα−/− mice (G), whereas FDC clusters were absent in the spleens of LTα−/− mice that received splenocytes from either wild-type (F) or LTα−/− mice (H).
Figure 5
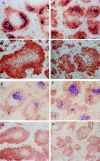
Spleen follicle structure in recipients of wild-type or LTα−/− bone marrow. 6 wk after bone marrow reconstitution, mice were immunized i.p. with SRBC and 10 d later sections of frozen spleen were stained with 8C12 (blue) to detect FDC, and with anti-B220 (brown). Clusters of FDC were detected in both wild-type mice (A) and LTα−/− mice (B) that had been reconstituted with wild-type BM. Clusters of FDC were not detected in either wild-type (C) or LTα−/− mice (D) that had received BM from LTα−/− mice. The GC reaction was assessed by staining spleen sections with PNA (blue) and anti-IgD (brown). GC were observed in both wild-type mice (E) and LTα−/− mice (F) that had received BM from wildtype donors, but were not detected in either wild-type (G) or LTα−/− mice (H) that were reconstituted with BM from LTα−/− donors.
Figure 6
B cell/T cell organization in irradiated mice reconstituted with wild-type or LTα−/− bone marrow. Frozen sections of spleens from the recipients of BM transfer shown in Figure 5 were analyzed by staining with anti–Thy-1.2 (blue) and anti-B220 (brown). Wild-type mice that received BM from either wild-type (A) or LTα−/− mice (B) showed segregation of B cells and T cells within lymphoid follicles, whereas the follicles of LTα−/− mice reconstituted with BM from either wild-type (C) or LTα−/− mice (D) showed little or no segregation of B and T cell zones.
Figure 7
IgG production after bone marrow transfer. Wild-type and LTα−/− mice (3–4 mice/group) were lethally irradiated and reconstituted with wild-type or LTα−/− as in Fig. 5. 6 wk later, they were immunized i.p. with SRBC (108) and boosted 21 d later. Serum was collected 10 d after both primary and secondary immunization and anti-SRBC IgG was measured by ELISA as in Fig. 3. Results represent means ± SEM. Similar results were obtained in two additional experiments.
Similar articles
- B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion.
Fu YX, Huang G, Wang Y, Chaplin DD. Fu YX, et al. J Exp Med. 1998 Apr 6;187(7):1009-18. doi: 10.1084/jem.187.7.1009. J Exp Med. 1998. PMID: 9529317 Free PMC article. - Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling.
Le Hir M, Bluethmann H, Kosco-Vilbois MH, Müller M, di Padova F, Moore M, Ryffel B, Eugster HP. Le Hir M, et al. J Exp Med. 1996 May 1;183(5):2367-72. doi: 10.1084/jem.183.5.2367. J Exp Med. 1996. PMID: 8642347 Free PMC article. - Antigen persistence is required for somatic mutation and affinity maturation of immunoglobulin.
Wang Y, Huang G, Wang J, Molina H, Chaplin DD, Fu YX. Wang Y, et al. Eur J Immunol. 2000 Aug;30(8):2226-34. doi: 10.1002/1521-4141(2000)30:8<2226::AID-IMMU2226>3.0.CO;2-5. Eur J Immunol. 2000. PMID: 10940914 - Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers.
Matsumoto M, Fu YX, Molina H, Chaplin DD. Matsumoto M, et al. Immunol Rev. 1997 Apr;156:137-44. doi: 10.1111/j.1600-065x.1997.tb00965.x. Immunol Rev. 1997. PMID: 9176705 Review.
Cited by
- Structure-modifying capacity of anti-tumour necrosis factor-alpha therapy in ankylosing spondylitis.
De Keyser F, Baeten D, Van den Bosch F, Kruithof E, Verbruggen G, Mielants H, Veys E. De Keyser F, et al. Drugs. 2004;64(24):2793-811. doi: 10.2165/00003495-200464240-00005. Drugs. 2004. PMID: 15563249 Review. - BOB.1/OBF.1 deficiency affects marginal-zone B-cell compartment.
Samardzic T, Marinkovic D, Nielsen PJ, Nitschke L, Wirth T. Samardzic T, et al. Mol Cell Biol. 2002 Dec;22(23):8320-31. doi: 10.1128/MCB.22.23.8320-8331.2002. Mol Cell Biol. 2002. PMID: 12417733 Free PMC article. - Naturally occurring B-cell responses to breast cancer.
Coronella-Wood JA, Hersh EM. Coronella-Wood JA, et al. Cancer Immunol Immunother. 2003 Dec;52(12):715-38. doi: 10.1007/s00262-003-0409-4. Epub 2003 Aug 15. Cancer Immunol Immunother. 2003. PMID: 12920480 Free PMC article. Review.
References
- Ruddle NH. Tumor necrosis factor (TNF-α) and lymphotoxin (TNFβ) Curr Opin Immunol. 1992;4:327–332. - PubMed
- Ware CF, VanArsdale TL, Crowe PD, Browning JL. The ligands and receptors of the lymphotoxin system. Curr Topics Microbiol Immunol. 1995;198:175–218. - PubMed
- Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O'Brine-Greco B, Foley SF, Ware CF. Lymphotoxin β, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. - PubMed
- Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-β-specific receptor. Science (Wash DC) 1994;264:707–710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous