Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments - PubMed (original) (raw)
Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments
J Chen et al. J Neurosci. 1997.
Abstract
Fos family transcription factors are believed to play an important role in the transcriptional responses of the brain to a variety of stimuli. Previous studies have described 35 and 37 kDa Fos-like proteins, termed chronic Fos-related antigens (FRAs), that are induced in brain in a region-specific manner in response to several chronic perturbations, including chronic electroconvulsive seizures, psychotropic drug treatments, and lesions. We show in this study that the chronic FRAs are isoforms of deltaFosB, a truncated splice variant of FosB that accumulate in brain after chronic treatments because of their stability. doffaFosB cDNA encodes the expression of 33, 35, and 37 kDa proteins that arise from a single AUG translation start site. The 35 and 37 kDa proteins correspond to the chronic FRAs that are induced in brain by chronic treatments, whereas the 33 kDa protein corresponds to a Fos-like protein that is induced in brain by acute treatments, findings based on migration on one- and two-dimensional Western blots with anti-FRA and anti-FosB antibodies. Using cells in which deltaFosB or FosB expression is under the control of a tetracycline-regulated gene expression system, we show that the 37 kDa deltaFosB protein exhibits a remarkably long half-life, the 35 kDa DeltaFosB protein exhibits an intermediate half-life, and the 33 kDa deltaFosB protein and all FosB-derived proteins exhibit relatively short half-lives. Moreover, we show that the 33 kDa deltaFosB protein is the first to appear after activation of deltaFosB expression. Finally, deltaFosB proteins are shown to possess DNA-binding activity and to exert potent transactivating effects in reporter gene assays. Together, these findings support a scheme wherein deltaFosB, expressed as a 33 kDa protein, is modified to form highly stable isoforms of 35 and 37 kDa. As a result, these stable isoforms gradually accumulate in the brain with repeated treatments to mediate forms of long-lasting neural and behavioral plasticity.
Figures
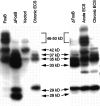
Fig. 1.
Western blots showing expression of FosB- and ΔFosB-derived proteins in stably transfected C6 glioma cells (left) and in transiently transfected Cath.a cells (right). Cells constitutively expressing FosB or ΔFosB cDNA were prepared. Cell extracts of these lines and of nontransfected cells (vector) were analyzed by Western blotting using the pan-FRA antibody (see Materials and Methods), which recognizes a domain conserved in all Fos-like proteins. Aliquots of cerebral cortex from rats treated acutely or chronically with ECS were analyzed for comparison. In both types of cells, FosB cDNA encodes the expression of proteins at 46–50 and 35 kDa. ΔFosB cDNA encodes the expression of proteins at 37, 35, 33, 29, and 28 kDa. The 35 and 37 kDa ΔFosB-encoded proteins comigrate with the 35 and 37 kDa chronic FRAs induced by chronic ECS treatment. The 33 kDa ΔFosB-encoded protein comigrates with the 33 kDa acute FRA induced by acute ECS treatment. The 29 and 28 kDa ΔFosB-encoded proteins do not correspond to FRAs detected in the brain; they are distinct from an ∼30 kDa protein seen in the chronic ECS sample that is constitutive and not regulated by ECS treatment. Note that a prominent ∼42 kDa protein (possibly FRA-1 or -2) is present in control C6 cells (vector1) and in the FosB transfectants but is not in the ΔFosB transfectants. Note also that an ∼40 kDa protein (possibly FRA-1 or -2) is induced in cortex by acute ECS treatment only. The results shown are representative of at least four separate determinations. All of the bands recognized by the anti-FRA antibody represent specific labeling, because detection was abolished by preincubation of the antibody with the immunizing peptide antigen (see Hope et al., 1994a).
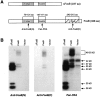
Fig. 2.
Western blots showing the expression of FosB- and ΔFosB-derived proteins in stably transfected C6 glioma cells.A, Regions of the ΔFosB and FosB proteins against which the anti-FosB(N), anti-FosB(C), and pan-FRA antibodies are directed. B, Extracts of stable cell lines (see Fig. 1) that allow constitutive expression of FosB or ΔFosB cDNA or extracts of nontransfected cells (Vector), analyzed by Western blotting using an antibody directed against the N terminus of FosB [anti-FosB(N)], an antibody directed against the C terminus of FosB [anti-FosB(C)], or the pan-FRA antibody. Note that all of the ΔFosB-derived proteins were recognized by the anti-FosB(N) antibody but not by the anti-FosB(C) antibody, whereas the FosB-derived proteins were recognized by both antibodies. Note also that none of the several proteins expressed in control cells and recognized by the pan-FRA antibody were recognized by the anti-FosB antibodies. The results shown are representative of three separate determinations.
Fig. 3.
Two-dimensional Western blots showing the expression of chronic FRAs in the brain and of ΔFosB-derived proteins in cultured cells. A, CATH.a cells transiently transfected with ΔFosB cDNA. B, Aliquots of cerebral cortex from rats treated chronically with ECSs, showing the migration of the 35 and 37 kDa chronic FRAs as demonstrated previously (Hope et al., 1994a; Chen et al., 1995). C, C6 glioma cells stably transfected with ΔFosB cDNA. Cell and brain extracts were analyzed by two-dimensional Western blotting using the pan-FRA antibody. The figure shows the comigration of the 37 kDa ΔFosB with the 37 kDa chronic FRA and of the 35 kDa ΔFosB with the 35 kDa chronic FRA. The 33 kDa ΔFosB comigrates with a 33 kDa FRA induced in the brain by an acute ECS treatment (see Chen et al., 1995). The results shown are representative of two to three separate determinations.
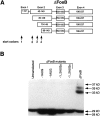
Fig. 4.
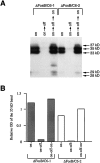
Analysis of translation start sites for ΔFosB-derived proteins. A, Genomic map for ΔFosB and the location of four alternative start (AUG) codons present in the resulting mRNA. B, Western blot of extracts of CATH.a cells transiently transfected with full-length ΔFosB cDNA or with mutants lacking the first (−1AUG) or the first and second (−1,2AUGs) start codons. Note that deletion of the first AUG completely obliterated the 37, 35, and 33 kDa ΔFosB proteins, whereas deletion of the first and second AUGs did not abolish the 28–29 kDa ΔFosB proteins, as indicated by the still higher levels of immunoreactivity present in this region of the gel compared with that in untransfected cells. The results shown are representative of three separate determinations.
Fig. 5.
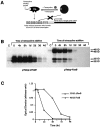
Analysis of the stability of ΔFosB and FosB proteins in CATH.a cells transiently transfected with ΔFosB or FosB cDNA under the control of the tetracycline expression system.A, Schematic illustration of the tetracycline-regulated gene expression system, as adapted from Dr. Rene Hen (Columbia University, Neuroscience Short Course, Society for Neuroscience, 1996).B, Western blot of ΔFosB and FosB proteins in CATH.a cells 24 hr after transfection (time 0) and at varying times after the addition of tetracycline (4 hr–4 d) to turn off expression.C, Quantitative representation of the time-dependent reduction in the 35 kDa ΔFosB protein and the 46 kDa FosB protein after the addition of tetracycline. The results shown are representative of two separate determinations.
Fig. 6.
Western blots showing the expression of ΔFosB proteins in C6 glioma cells stably transfected with ΔFosB cDNA under the control of the tetracycline expression system. A, Western blot analysis, using the pan-FRA antibody, of extracts of stable C6 glioma cell lines. B, Quantitative representation of the Western blots. Results from two independent stable cell lines are shown. One set of cells was harvested after being cultured for 3 d in the absence of tetracycline to turn ΔFosB expression on (on); a second set of cells was harvested after being cultured for 3 d in the absence of tetracycline, followed by 1 wk in the presence of tetracycline to turn ΔFosB expression off (on → off); a third set of cells was harvested after being cultured for 3 d in the absence of tetracycline, followed by 1 wk in the presence of tetracycline and then 1 wk in the absence of tetracycline to turn ΔFosB expression back on (on → off → on). Note the ability to turn ΔFosB expression repeatedly on and off by use of the tetracycline expression system. Note also that the 37 kDa ΔFosB protein appears appreciably only after more prolonged periods of expression (i.e., in the on → off → on condition). The results shown are representative of two separate determinations of six independent cell lines examined.
Fig. 7.
Western blot showing the relative stability of the 37, 35, and 33 kDa ΔFosB proteins in stable C6 glioma cells transfected with ΔFosB cDNA under the control of the tetracycline expression system. Cells were grown for varying times in the absence or presence of tetracycline to turn ΔFosB expression on (+) or off (−), respectively, and cell extracts were then analyzed by Western blotting using the pan-FRA antibody. In lane 1, cells were harvested after being grown in the absence of tetracycline for 11 d. In lane 2, cells were harvested after being grown in the absence of tetracycline for 5 d, followed by an additional 6 d in the presence of tetracycline. In lane 3, cells were harvested after being grown in the absence of tetracycline for 5 d, followed by 3 d in the presence of tetracycline and another 3 d in the absence of tetracycline. In lane 4, cells were harvested after being grown in the absence of tetracycline for 8 d, followed by an additional 3 d in the presence of tetracycline. Control (untransfected) cells are shown for comparison and illustrate the presence of an endogenous FRA, possibly FRA-2, of ∼42 kDa (see also Fig. 1). The results demonstrate that the 33 kDa ΔFosB protein is the first to appear after activation of ΔFosB expression, but this protein is less stable compared with the 35 and 37 kDa ΔFosB proteins. The results shown are representative of three separate determinations using two independent, stable cell lines.
Fig. 8.
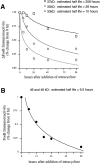
Analysis of the stability of ΔFosB and FosB proteins in stable C6 glioma cells transfected with ΔFosB or FosB cDNAs under the control of the tetracycline expression system. Cells were grown in the absence of tetracycline for 11 d to turn ΔFosB (A) or FosB (B) expression on, after which time the cells were cultured in the presence of tetracycline for varying periods to turn expression off. The results demonstrate the dramatic stability of the 37 kDa ΔFosB protein compared with the other ΔFosB and FosB proteins. The results shown are representative of two separate determinations using two independent, stable cell lines.
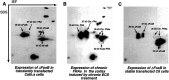
Fig. 9.
Gel shift assays showing the induction of AP-1 DNA-binding activity after the expression of ΔFosB or FosB in stable C6 glioma cells. Extracts of a stable cell line that allows constitutive expression of ΔFosB (A) or FosB (B) cDNA and of cells transfected with vector DNA only (Vector control) were analyzed for AP-1 DNA-binding activity using gel shift assays. Extracts of cerebral cortex from rats that were treated acutely or chronically with ECSs, or with sham treatments, were analyzed for comparison. As shown in_A_, the expression of ΔFosB results in the dramatic induction of an AP-1 complex that comigrates with the AP-1 complex induced by chronic, but not acute, ECSs. In contrast, as shown in_B_, the expression of FosB results in the induction of an AP-1 complex that comigrates with the AP-1 complex induced in brain by acute ECS. The results shown are representative of three separate determinations.
Fig. 10.
Analysis of the transactivating properties of ΔFosB and FosB proteins in SH-SY5Y and C6 glioma cells.A, Results from SH-SY5Y cells transiently transfected with ΔFosB cDNA under the control of the tetracycline expression system. B, C, Results from C6 glioma cells stably transfected with ΔFosB and FosB, respectively, under the control of the tetracycline expression system. Both types of cells were also transiently transfected with 4×AP-1/RSV-Luc, which contains a promoter of four tandem consensus AP-1 sites driving the expression of a luciferase reporter gene. The results demonstrate the ability of ΔFosB to upregulate AP-1 promoter activity in both cell lines. In contrast, FosB downregulates AP-1 promoter activity in the C6 cells. The results shown are representative of three separate determinations.
Similar articles
- Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments.
Chen J, Nye HE, Kelz MB, Hiroi N, Nakabeppu Y, Hope BT, Nestler EJ. Chen J, et al. Mol Pharmacol. 1995 Nov;48(5):880-9. Mol Pharmacol. 1995. PMID: 7476919 - Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum.
Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St-Jean M, Wigle N, Blanchet P, Grondin R, Robertson GS. Doucet JP, et al. Eur J Neurosci. 1996 Feb;8(2):365-81. doi: 10.1111/j.1460-9568.1996.tb01220.x. Eur J Neurosci. 1996. PMID: 8714707 - Regulation of fosB and DeltafosB mRNA expression: in vivo and in vitro studies.
Alibhai IN, Green TA, Potashkin JA, Nestler EJ. Alibhai IN, et al. Brain Res. 2007 Apr 27;1143:22-33. doi: 10.1016/j.brainres.2007.01.069. Epub 2007 Jan 27. Brain Res. 2007. PMID: 17324382 Free PMC article. - DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity.
Nestler EJ, Kelz MB, Chen J. Nestler EJ, et al. Brain Res. 1999 Jul 17;835(1):10-7. doi: 10.1016/s0006-8993(98)01191-3. Brain Res. 1999. PMID: 10448191 Review. - ∆FosB: a transcriptional regulator of stress and antidepressant responses.
Nestler EJ. Nestler EJ. Eur J Pharmacol. 2015 Apr 15;753:66-72. doi: 10.1016/j.ejphar.2014.10.034. Epub 2014 Nov 7. Eur J Pharmacol. 2015. PMID: 25446562 Free PMC article. Review.
Cited by
- Periadolescent mice show enhanced DeltaFosB upregulation in response to cocaine and amphetamine.
Ehrlich ME, Sommer J, Canas E, Unterwald EM. Ehrlich ME, et al. J Neurosci. 2002 Nov 1;22(21):9155-9. doi: 10.1523/JNEUROSCI.22-21-09155.2002. J Neurosci. 2002. PMID: 12417638 Free PMC article. - Homeostatic state of microglia in a rat model of chronic sleep restriction.
Hall S, Deurveilher S, Robertson GS, Semba K. Hall S, et al. Sleep. 2020 Nov 12;43(11):zsaa108. doi: 10.1093/sleep/zsaa108. Sleep. 2020. PMID: 32474610 Free PMC article. - ΔFosB induction correlates inversely with CB₁ receptor desensitization in a brain region-dependent manner following repeated Δ⁹-THC administration.
Lazenka MF, Selley DE, Sim-Selley LJ. Lazenka MF, et al. Neuropharmacology. 2014 Feb;77:224-33. doi: 10.1016/j.neuropharm.2013.09.019. Epub 2013 Sep 30. Neuropharmacology. 2014. PMID: 24090766 Free PMC article. - Essential role of the fosB gene in molecular, cellular, and behavioral actions of chronic electroconvulsive seizures.
Hiroi N, Marek GJ, Brown JR, Ye H, Saudou F, Vaidya VA, Duman RS, Greenberg ME, Nestler EJ. Hiroi N, et al. J Neurosci. 1998 Sep 1;18(17):6952-62. doi: 10.1523/JNEUROSCI.18-17-06952.1998. J Neurosci. 1998. PMID: 9712664 Free PMC article. - Functional role of the N-terminal domain of ΔFosB in response to stress and drugs of abuse.
Ohnishi YN, Ohnishi YH, Vialou V, Mouzon E, LaPlant Q, Nishi A, Nestler EJ. Ohnishi YN, et al. Neuroscience. 2015 Jan 22;284:165-170. doi: 10.1016/j.neuroscience.2014.10.002. Epub 2014 Oct 13. Neuroscience. 2015. PMID: 25313003 Free PMC article.
References
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human blastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. - PubMed
- Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. - PubMed
- Campeau S, Hayward MD, Hope B, Rosen JB, Nestler EJ, Davis M. Induction of c-fos proto-oncogene in rat amygdala during unconditioned and conditioned fear. Brain Res. 1991;565:349–352. - PubMed
- Chen J, Nye HE, Kelz MB, Hiroi N, Nakabeppu Y, Hope BT, Nestler EJ. Regulation of dFosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol Pharmacol. 1995;48:880–889. - PubMed
- Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St-Jean M, Wigle N, Planchet P, Grondin R, Robertson GS. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of dFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci. 1996;8:365–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH25642/MH/NIMH NIH HHS/United States
- R01 DA007359/DA/NIDA NIH HHS/United States
- DA07359/DA/NIDA NIH HHS/United States
- P01 MH025642/MH/NIMH NIH HHS/United States
- MH51399/MH/NIMH NIH HHS/United States
- R37 DA007359/DA/NIDA NIH HHS/United States
- R01 MH051399/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous