Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2 - PubMed (original) (raw)
Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2
M Furuta et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The prohormone convertase SPC2 (PC2) participates in the processing of proinsulin, proglucagon, and a variety of other neuroendocrine precursors, acting either alone or in conjunction with the structurally related dense-core granule convertase SPC3 (PC3/PC1). We have generated a strain of mice lacking active SPC2 by introducing the neomycin resistance gene (Neor) into the third exon of the mSPC2 gene. This gene insertion results in the synthesis of an exon 3-deleted form of SPC2 that does not undergo autoactivation and is not secreted. The homozygous mutant mice appear to be normal at birth. However, they exhibit a small decrease in rate of growth. They also have chronic fasting hypoglycemia and a reduced rise in blood glucose levels during an intraperitoneal glucose tolerance test, which is consistent with a deficiency of circulating glucagon. The processing of proglucagon, prosomatostatin, and proinsulin in the alpha, delta, and beta cells, respectively, of the pancreatic islets is severely impaired. The islets in mutant mice at 3 months of age show marked hyperplasia of alpha and delta cells and a relative diminution of beta cells. SPC2-defective mice offer many possibilities for further delineating neuroendocrine precursor processing mechanisms and for exploring more fully the physiological roles of many neuropeptides and peptide hormones.
Figures
Figure 1
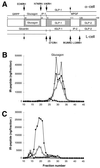
Gene targeting scheme for disruption of the mouse SPC2 locus. (A) Schematic representation of the mouse SPC2 protein. The signal sequence (PRE), proregion (PRO), catalytic domain (CAT), P domain (P), and predicted amphipathic helical region (AH) are shaded as indicated. (B) Strategy for replacement of a segment of the SPC2 gene by homologous recombination. The exon–intron junctions in the mouse gene corresponded to those in the human gene (38). The primary cleavage site for activation (PCS) and a secondary cleavage site (SCS) are indicated by vertical arrows.
Figure 2
Western blot analysis of islets for SPC2 expression. In wild-type islets, 75-kDa proSPC2 and 64-kDa mature SPC2 were demonstrated (lane 1), whereas in SPC2−/− islets, only the mutant SPC2ΔExon3 protein of 72 kDa was demonstrated (lane 2). Fifty islets were isolated from 3-month-old SPC2−/− or SPC2+/+ mice, and the proteins were resolved on 7.5% SDS/PAGE, transferred to Immunobilon-P (Amersham), incubated with antiserum PEP4 (immunogenic peptide: residues 611–635 of mouse SPC2; diluted 1:10,000), and detected by ECL (Amersham).
Figure 3
Biosynthesis of normal and mutant SPC2 in cultured cells. HEK293 cells or AtT-20 cells were stably transfected with constructs encoding wild-type SPC2 or mutant SPC2 (SPC2ΔExon3) (39). Cells were labeled with [35S]methionine [1 mCi/ml; 1,000 Ci/mmol; 1 Ci = 37 GBq] for 30 min and then chase-incubated in nonradioactive medium for 3 hr at 37°C. At the termination of the incubation, media were collected and cells were lysed with a _N_-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid/Mannitol buffer (40). Cell extracts and media samples were immunoprecipitated with SPC2 C-terminal antiserum and then analyzed by SDS/PAGE followed by fluorography. The mutant protein was not cleaved or secreted, in contrast to wild-type SPC2. To investigate whether the lack of processing activity of SPC2ΔExon3 in HEK293 cells was due to the lack of 7B2, a neuroendocrinal-specific protein that is involved in the transport and activation of SPC2 (41, 42), wild-type SPC2 and SPC2ΔExon3 were also stably transfected into AtT-20 cells, a corticotrope cell line that endogenously expresses 7B2. Pulse–chase metabolic labeling yielded similar results in this endocrine cell line. SPC2ΔExon3 appeared to be unstable and to undergo more rapid intracellular degradation without detectable secretion. P, pulse cell lysates; C, chase cell lysates; M, chase media.
Figure 4
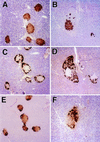
Immunohistochemical staining of islets of normal (A, C, and E) and mutant (B, D, and F) mice. A and B show islets stained with an insulin antibody that crossreacts with proinsulin. C and D show islets stained with a glucagon antibody that crossreacts with proglucagon, and E and F show islets stained with somatostatin antibody that crossreacts with prosomatostatin. A positive reaction is indicated by the cytoplasmic accumulation of brown reaction product. (Magnification = ×145.)
Figure 5
Differential processing of proglucagon in the normal pancreas (α-cell) and the intestine (L-cell), and results of radioimmunoassay of glucagon in pancreas extracts. (A) Schematic representation of the structure and processing of proglucagon (20, 24, 25). At the top are the peptides resulting from proglucagon processing at the sites indicated in the pancreatic alpha cell. At the bottom are the peptides resulting from processing at the indicated sites in the intestinal L cell. These peptides include the glicentin-related polypeptide (GRPP, [proglucagon-(1–30)]), glucagon [proglucagon-(33–61)], the intervening peptide 1 (IP-1, [proglucagon-(64–69)]), the major proglucagon fragment (MPGF, [proglucagon-(72–158)]), GLP-1 [proglucagon-(72–107)], glicentin [proglucagon-(1–69)], truncated GLP-1 (tGLP-1, [proglucagon-(78–107)]), intervening peptide 2 (IP-2, [proglucagon-(111–122)]), GLP-2 [proglucagon-(126–158)], and oxyntomodulin [proglucagon-(33–69)]. The cleavage sites are indicated by arrows, with the sequence surrounding the site shown above or below the arrow. (Note that all cleavages are to the right of residues shown in bold.) Partially processed sites are indicated by small arrows. B and C show gel filtration profiles of extracts of wild-type (B) and SPC2−/− (C) pancreas analyzed using antibodies against four different regions of proglucagon. Antiserum 4304 (•) recognizes the 6–15 sequence of glucagon; 4305 (○) recognizes only the free, unextended form of glucagon; 4830 (□) was raised against the N terminus of glucagon, but shows some crossreaction with N-terminally extended forms of glucagon; 2135 (▴) recognizes a C-terminal immunodeterminant of GLP-1 and also reacts with MPGF (43).
Figure 6
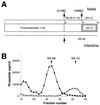
Radioimmunoassay of somatostatin-related peptides in pancreas extracts. (A) Schematic depiction of islet vs. intestinal processing of prosomatostatin (44). Indicated peptides are prosomatostatin 1–64, somatostatin 28 [SS-28, prosomatostatin-(65–92)], somatostatin 28(1–12) [prosomatostatin-(65–76)], and somatostatin 14 [SS-14, prosomatostatin-(79–92)]. The cleaved sites are indicated by arrows, with the sequence surrounding the site shown above or below the arrow. (B) Gel filtration of extracts of wild-type and SPC2−/− pancreas analyzed by radioimmunoassay for somatostatin (RIK 8001; Peninsula Laboratories). This somatostatin antibody crossreacts with both SS-28 and prosomatostatin. ○ indicate wild-type extract and • indicate SPC2−/− pancreas extract. The arrows show the elution positions of SS-28 and SS-14.
Figure 7
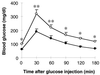
Glucose tolerance tests. Glucose tolerance tests were carried out on 8-week-old wild-type (○) and SPC2−/− (•) mice after fasting for 20 hr. Results are mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.01 (Student’s t test).
Similar articles
- Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2.
Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. Furuta M, et al. J Biol Chem. 1998 Feb 6;273(6):3431-7. doi: 10.1074/jbc.273.6.3431. J Biol Chem. 1998. PMID: 9452465 - Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide.
Rouillé Y, Martin S, Steiner DF. Rouillé Y, et al. J Biol Chem. 1995 Nov 3;270(44):26488-96. doi: 10.1074/jbc.270.44.26488. J Biol Chem. 1995. PMID: 7592866 - Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice.
Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, Steiner DF. Furuta M, et al. J Biol Chem. 2001 Jul 20;276(29):27197-202. doi: 10.1074/jbc.M103362200. Epub 2001 May 16. J Biol Chem. 2001. PMID: 11356850 - Altered islet prohormone processing: a cause or consequence of diabetes?
Ramzy A, Kieffer TJ. Ramzy A, et al. Physiol Rev. 2022 Jan 1;102(1):155-208. doi: 10.1152/physrev.00008.2021. Epub 2021 Jul 19. Physiol Rev. 2022. PMID: 34280055 Review. - Islet prohormone processing in health and disease.
Chen YC, Taylor AJ, Verchere CB. Chen YC, et al. Diabetes Obes Metab. 2018 Sep;20 Suppl 2:64-76. doi: 10.1111/dom.13401. Diabetes Obes Metab. 2018. PMID: 30230179 Review.
Cited by
- Is GLP-1 a hormone: Whether and When?
D'Alessio D. D'Alessio D. J Diabetes Investig. 2016 Apr;7 Suppl 1(Suppl 1):50-5. doi: 10.1111/jdi.12466. Epub 2016 Mar 14. J Diabetes Investig. 2016. PMID: 27186356 Free PMC article. Review. - Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development.
Stoffel W, Jenke B, Blöck B, Zumbansen M, Koebke J. Stoffel W, et al. Proc Natl Acad Sci U S A. 2005 Mar 22;102(12):4554-9. doi: 10.1073/pnas.0406380102. Epub 2005 Mar 11. Proc Natl Acad Sci U S A. 2005. PMID: 15764706 Free PMC article. - Deletion of the glucagon receptor gene before and after experimental diabetes reveals differential protection from hyperglycemia.
Rivero-Gutierrez B, Haller A, Holland J, Yates E, Khrisna R, Habegger K, Dimarchi R, D'Alessio D, Perez-Tilve D. Rivero-Gutierrez B, et al. Mol Metab. 2018 Nov;17:28-38. doi: 10.1016/j.molmet.2018.07.012. Epub 2018 Aug 20. Mol Metab. 2018. PMID: 30170980 Free PMC article. - Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing.
Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L, Patterson SD, Courchesne PL, Richards B, Levin N, Mzhavia N, Devi LA, Douglass J. Fricker LD, et al. J Neurosci. 2000 Jan 15;20(2):639-48. doi: 10.1523/JNEUROSCI.20-02-00639.2000. J Neurosci. 2000. PMID: 10632593 Free PMC article. - Proteases for processing proneuropeptides into peptide neurotransmitters and hormones.
Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Hook V, et al. Annu Rev Pharmacol Toxicol. 2008;48:393-423. doi: 10.1146/annurev.pharmtox.48.113006.094812. Annu Rev Pharmacol Toxicol. 2008. PMID: 18184105 Free PMC article. Review.
References
- Steiner D F, Smeekens S P, Ohagi S, Chan S J. J Biol Chem. 1992;267:23435–23438. - PubMed
- Seidah N G, Chrétien M. Trends Endocrinol Metab. 1992;3:133–140. - PubMed
- Bailyes E M, Bennett D L, Hutton J C. Enzyme (Basel) 1991;45:301–313. - PubMed
- Seidah N G, Chrétien M, Day R. Biochimie. 1994;76:197–209. - PubMed
- Hook V Y H, Azaryan A V, Hwang S-R, Tezapsidis N. FASEB J. 1994;8:1269–1278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK013914/DK/NIDDK NIH HHS/United States
- DK13914/DK/NIDDK NIH HHS/United States
- DK20595/DK/NIDDK NIH HHS/United States
- P30 DK020595/DK/NIDDK NIH HHS/United States
- P60 DK020595/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous