A "knockdown" mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1 - PubMed (original) (raw)
A "knockdown" mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1
M A McDevitt et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The hematopoietic-restricted transcription factor GATA-1 is required for both mammalian erythroid cell and megakaryocyte differentiation. To define the mechanisms governing its transcriptional regulation, we replaced upstream sequences including a DNase I hypersensitive (HS) region with a neomycin-resistance cassette by homologous recombination in mouse embryonic stem cells and generated mice either harboring this mutation (neoDeltaHS) or lacking the selection cassette (DeltaneoDeltaHS). Studies of the consequences of these targeted mutations provide novel insights into GATA-1 function in erythroid cells. First, the neoDeltaHS mutation leads to a marked impairment in the rate or efficiency of erythroid cell maturation due to a modest (4- to 5-fold) decrease in GATA-1 expression. Hence, erythroid differentiation is dose-dependent with respect to GATA-1. Second, since expression of GATA-1 from the DeltaneoDeltaHS allele in erythroid cells is largely restored, transcription interference imposed by the introduced cassette must account for the "knockdown" effect of the mutation. Finally, despite the potency of the upstream sequences in conferring high-level, developmentally appropriate expression of transgenes in mice, other cis-regulatory elements within the GATA-1 compensate for its absence in erythroid cells. Our work illustrates the usefulness of targeted mutations to create knockdown mutations that may uncover important quantitative contributions of gene function not revealed by conventional knockouts.
Figures
Figure 1
Structures of wild-type, neoΔHS, and ΔneoΔHS GATA-1 alleles. The wild-type locus contains alternative first exons (IT and IE) and a DNase I HS region (10). Exons are indicated by solid boxes. In the neoΔHS locus a loxP-flanked neomycin-resistance cassette replaces ≈8 kb lying between a _Bam_HI site (B) and an artificial _Xho_I site (〈Xh〉) present in a λ phage genomic clone used in the construction. Cre-mediated excision of the neomycin-resistance cassette leaves a single loxP site (indicated by the solid triangle) in the ΔneoΔHS locus.
Figure 2
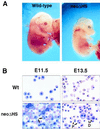
Anemia and abnormal erythropoiesis in neoΔHS embryos. (A) Embryos at E13.5. (B) Peripheral blood smears at yolk sac (E11.5) and fetal liver (E13.5) stages. Note binucleate primitive erythrocytes, indicated by the arrows on the left and arrows with P on the right. Note the presence of immature definitive erythroid cells (arrows with D) at E13.5.
Figure 3
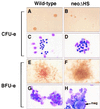
Impaired erythroid colony formation of neoΔHS fetal liver cells. CFU-e and BFU-e colonies were cultured as described. Note the small size and pallor of mutant CFU-e colonies (A and B) and the increased nuclear/cytoplasmic ratio of stained erythroid cells (C and D). neoΔHS BFU-e are larger in size and less red in appearance (E and F). BFU-e contain less mature and dysplastic erythroid precursors and abundant megakaryocytes (G and H).
Figure 4
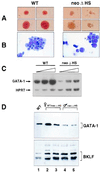
Decreased GATA-1 RNA and protein levels in neoΔHS erythroid cells. (A and B) Appearance of representative definitive erythroid colonies and cells obtained by in vitro differentiation of ES cells. Colonies were harvested at day 5 after disaggregation of embryoid bodies. Note the pallor of mutant colonies and the predominance of immature erythroid cells. (C) Semiquantitative RT-PCR analysis of GATA-1 RNA transcripts. Assays were performed as described (9) with 28, 30, and 32 cycles of PCR (shown by triangles) and primer pairs specific for GATA-1 and hypoxanthine phosphoribosyltransferase (HPRT) (as an internal control). Relative band intensities were quantitated by PhosphorImager analysis. (D). Western blot analysis of fetal liver erythroid cell nuclear proteins. Wild-type female (lane 1), heterozygous neoΔHS female (lanes 2 and 3), and hemizygous neoΔHS males (lanes 4 and 5) are indicated.
Similar articles
- Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line.
Weiss MJ, Yu C, Orkin SH. Weiss MJ, et al. Mol Cell Biol. 1997 Mar;17(3):1642-51. doi: 10.1128/MCB.17.3.1642. Mol Cell Biol. 1997. PMID: 9032291 Free PMC article. - Different sequence requirements for expression in erythroid and megakaryocytic cells within a regulatory element upstream of the GATA-1 gene.
Vyas P, McDevitt MA, Cantor AB, Katz SG, Fujiwara Y, Orkin SH. Vyas P, et al. Development. 1999 Jun;126(12):2799-811. doi: 10.1242/dev.126.12.2799. Development. 1999. PMID: 10331989 - An upstream, DNase I hypersensitive region of the hematopoietic-expressed transcription factor GATA-1 gene confers developmental specificity in transgenic mice.
McDevitt MA, Fujiwara Y, Shivdasani RA, Orkin SH. McDevitt MA, et al. Proc Natl Acad Sci U S A. 1997 Jul 22;94(15):7976-81. doi: 10.1073/pnas.94.15.7976. Proc Natl Acad Sci U S A. 1997. PMID: 9223298 Free PMC article. - Transcription factor GATA-1 and erythroid development.
Simon MC. Simon MC. Proc Soc Exp Biol Med. 1993 Feb;202(2):115-21. doi: 10.3181/00379727-202-43519a. Proc Soc Exp Biol Med. 1993. PMID: 8424101 Review. - Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells.
Simon MC, Pevny L, Wiles MV, Keller G, Costantini F, Orkin SH. Simon MC, et al. Nat Genet. 1992 May;1(2):92-8. doi: 10.1038/ng0592-92. Nat Genet. 1992. PMID: 1302015 Review.
Cited by
- Molecular Mechanisms of the Genetic Predisposition to Acute Megakaryoblastic Leukemia in Infants With Down Syndrome.
Grimm J, Heckl D, Klusmann JH. Grimm J, et al. Front Oncol. 2021 Mar 11;11:636633. doi: 10.3389/fonc.2021.636633. eCollection 2021. Front Oncol. 2021. PMID: 33777792 Free PMC article. Review. - The CXCR1/CXCR2 Inhibitor Reparixin Alters the Development of Myelofibrosis in the Gata1 low Mice.
Verachi P, Gobbo F, Martelli F, Martinelli A, Sarli G, Dunbar A, Levine RL, Hoffman R, Massucci MT, Brandolini L, Giorgio C, Allegretti M, Migliaccio AR. Verachi P, et al. Front Oncol. 2022 Mar 22;12:853484. doi: 10.3389/fonc.2022.853484. eCollection 2022. Front Oncol. 2022. PMID: 35392239 Free PMC article. - HP1gamma function is required for male germ cell survival and spermatogenesis.
Brown JP, Bullwinkel J, Baron-Lühr B, Billur M, Schneider P, Winking H, Singh PB. Brown JP, et al. Epigenetics Chromatin. 2010 Apr 27;3(1):9. doi: 10.1186/1756-8935-3-9. Epigenetics Chromatin. 2010. PMID: 20423503 Free PMC article. - hGATA1 Under the Control of a μLCR/β-Globin Promoter Rescues the Erythroid but Not the Megakaryocytic Phenotype Induced by the Gata1 low Mutation in Mice.
Martelli F, Verachi P, Zingariello M, Mazzarini M, Vannucchi AM, Lonetti A, Bacci B, Sarli G, Migliaccio AR. Martelli F, et al. Front Genet. 2021 Oct 11;12:720552. doi: 10.3389/fgene.2021.720552. eCollection 2021. Front Genet. 2021. PMID: 34707640 Free PMC article. - Differential requirements for the activation domain and FOG-interaction surface of GATA-1 in megakaryocyte gene expression and development.
Muntean AG, Crispino JD. Muntean AG, et al. Blood. 2005 Aug 15;106(4):1223-31. doi: 10.1182/blood-2005-02-0551. Epub 2005 Apr 28. Blood. 2005. PMID: 15860665 Free PMC article.
References
- Orkin S H. Curr Opin Genet Dev. 1996;6:597–602. - PubMed
- Evans T, Felsenfeld G. Cell. 1989;58:877–885. - PubMed
- Martin D I K, Tsai S-F, Orkin S H. Nature (London) 1989;338:435–438. - PubMed
- Tsai S F, Martin D I, Zon L I, D’Andrea A D, Wong G G, Orkin S H. Nature (London) 1989;339:446–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases