The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation - PubMed (original) (raw)
The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation
P J Lehner et al. Proc Natl Acad Sci U S A. 1997.
Abstract
In its attempt to evade cytotoxic T cell recognition, human cytomegalovirus encodes several genes that target MHC class I molecules at different points in their assembly pathway. We show here that the human cytomegalovirus US6 gene encodes a 22-kDa glycoprotein that binds the transporter-associated with antigen processing (TAP)/class I complex and inhibits translocation of peptide from the cytosol to the endoplasmic reticulum. Major histocompatibility complex class I molecules are therefore unable to load TAP-dependent peptides, resulting in the retention of MHC class I molecules in the endoplasmic reticulum, with a consequent reduction in class I at the cell surface. Interferon-gamma treatment of US6 transfected cells overcomes this inhibition of peptide translocation and restores class I at the cell surface to wild type levels. The functional consequence of TAP inhibition is that US6 transfected cells are unable to present endogenous antigen to cytotoxic T lymphocytes and are therefore resistant to cytotoxic T lymphocyte lysis.
Figures
Figure 1
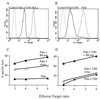
Expression of the US6 gene decreases cell surface expression of MHC class I molecules and prevents CTL recognition of virus-infected cells. US6 and control-transfected HeLaM cells (A) and PaLa cells (B) were analyzed for class I surface expression by flow cytometry using the mAb w6/32 and fluorescein-conjugated rabbit anti-mouse IgG. The control was a nonspecific isotype-matched monoclonal antibody. Peripheral blood mononuclear cells from an HLA-B8 positive donor were incubated in vitro with influenza A-infected cells and pulsed on day 8 with N380–88 peptide and feeder cells. On day 12 of bulk culture, CTL lysis was tested in a 51Cr release assay against Pala control (C) and Pala.US6-transfected (D) target cells, which were infected with influenza virus, pulsed with N380–88 peptide (10 μM), or untreated.
Figure 2
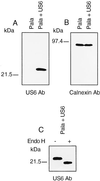
The US6 gene encodes a 22-kDa glycoprotein. US6 and control-transfected Pala cells were extracted in 1% Triton X-100 and lysates separated by SDS/12.5% PAGE, transferred to Immobilon-P membranes, and probed with anti-US6 (A) and anti-calnexin (B) antibodies. (C) Crude membrane extracts from US6 and control-transfected cells were prepared by freeze thawing. Membranes were extracted in endo H buffer, subjected to endo H or mock digestion, separated by SDS/PAGE, and after transfer to Immobilon-P membranes probed with antibody specific for US6.
Figure 3
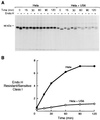
Transport of class I heavy chains is inhibited in US6-transfected cells. US6 and vector control-transfected HeLaM cells were radiolabeled with [35S]methionine and [35S]cysteine for 15 min and chased for the indicated time periods. Triton X-100 lysates (1%) were immunoprecipitated with the mAb w6/32, digested or mock-digested with endo H, and subjected to 10% SDS/PAGE (A), and the ratio of endo H-resistant vs. endo H-sensitive class I heavy chains present at each time point quantitated by image analysis (B).
Figure 4
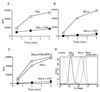
TAP-mediated peptide translocation is inhibited in US6-transfected cells and can be overcome with IFN-γ. Streptolysin-O (Murex)-permeabilized US6 and control-transfected Pala cells (A), HeLaM (B), and HeLaM cells that had been pretreated with IFN-γ (200 unit/ml) for 48 hr (C) were incubated with an iodinated reporter peptide at 37°C for the indicated time period, and the reaction was stopped by lysis with 3% Triton X-100, as described in Materials and Methods. Translocation into the ER was assessed by binding of the glycosylated reporter peptide to concanavalin A-Sepharose beads and counting on a γ-counter. HeLaM, US6-transfected HeLaM, and US6-transfected HeLaM cells that had been pretreated with IFN-γ were analyzed for class I surface expression by flow cytometry, using the mAb w6/32 and fluorescein-conjugated rabbit anti-mouse IgG (D). The control was a nonspecific isotype-matched monoclonal antibody.
Figure 5
US6 associates with the TAP/tapasin/class I complex. Digitonin lysates (1%) from US6 and control-transfected PaLa cells were immunoprecipitated with TAP1-specific (148.3) and control (κ immunoglobulin light chain-specific) mAb, coupled to A-15 m Sepharose beads. Precipitated proteins were eluted in 0.1% SDS/0.05% Triton X-100 buffer, separated by 12.5% SDS/PAGE under nonreducing conditions, transferred to Immobilon P membrane, and probed with TAP-specific, R.RING 4C (A), tapasin-specific, R.tapasinN (B), class I heavy chain-specific, 3B10.7 (C), and anti-US6 (D) antibodies.
Similar articles
- The human cytomegalovirus gene product US6 inhibits ATP binding by TAP.
Hewitt EW, Gupta SS, Lehner PJ. Hewitt EW, et al. EMBO J. 2001 Feb 1;20(3):387-96. doi: 10.1093/emboj/20.3.387. EMBO J. 2001. PMID: 11157746 Free PMC article. - Human cytomegalovirus UL18 utilizes US6 for evading the NK and T-cell responses.
Kim Y, Park B, Cho S, Shin J, Cho K, Jun Y, Ahn K. Kim Y, et al. PLoS Pathog. 2008 Aug 8;4(8):e1000123. doi: 10.1371/journal.ppat.1000123. PLoS Pathog. 2008. PMID: 18688275 Free PMC article. - Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules.
Jun Y, Kim E, Jin M, Sung HC, Han H, Geraghty DE, Ahn K. Jun Y, et al. J Immunol. 2000 Jan 15;164(2):805-11. doi: 10.4049/jimmunol.164.2.805. J Immunol. 2000. PMID: 10623826 - Involvement of autophagy in MHC class I antigen presentation.
Øynebråten I. Øynebråten I. Scand J Immunol. 2020 Nov;92(5):e12978. doi: 10.1111/sji.12978. Epub 2020 Oct 19. Scand J Immunol. 2020. PMID: 32969499 Free PMC article. Review. - Corking the bottleneck: the transporter associated with antigen processing as a target for immune subversion by viruses.
Momburg F, Hengel H. Momburg F, et al. Curr Top Microbiol Immunol. 2002;269:57-74. doi: 10.1007/978-3-642-59421-2_4. Curr Top Microbiol Immunol. 2002. PMID: 12224516 Review.
Cited by
- Viral evasion of the MHC class I antigen-processing machinery.
Loch S, Tampé R. Loch S, et al. Pflugers Arch. 2005 Dec;451(3):409-17. doi: 10.1007/s00424-005-1420-8. Epub 2005 Aug 6. Pflugers Arch. 2005. PMID: 16086162 Review. - Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing.
Koppers-Lalic D, Reits EA, Ressing ME, Lipinska AD, Abele R, Koch J, Marcondes Rezende M, Admiraal P, van Leeuwen D, Bienkowska-Szewczyk K, Mettenleiter TC, Rijsewijk FA, Tampé R, Neefjes J, Wiertz EJ. Koppers-Lalic D, et al. Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):5144-9. doi: 10.1073/pnas.0501463102. Epub 2005 Mar 25. Proc Natl Acad Sci U S A. 2005. PMID: 15793001 Free PMC article. - The human cytomegalovirus US8 glycoprotein binds to major histocompatibility complex class I products.
Tirabassi RS, Ploegh HL. Tirabassi RS, et al. J Virol. 2002 Jul;76(13):6832-5. doi: 10.1128/jvi.76.13.6832-6835.2002. J Virol. 2002. PMID: 12050396 Free PMC article. - Major histocompatibility complex class I-restricted alloreactive CD4+ T cells.
Boyle LH, Goodall JC, Gaston JS. Boyle LH, et al. Immunology. 2004 May;112(1):54-63. doi: 10.1111/j.1365-2567.2004.01857.x. Immunology. 2004. PMID: 15096184 Free PMC article. - Overcoming innate immune barriers that impede AAV gene therapy vectors.
Muhuri M, Maeda Y, Ma H, Ram S, Fitzgerald KA, Tai PW, Gao G. Muhuri M, et al. J Clin Invest. 2021 Jan 4;131(1):e143780. doi: 10.1172/JCI143780. J Clin Invest. 2021. PMID: 33393506 Free PMC article. Review.
References
- Reusser P, Riddell S R, Meyers J D, Greenberg P D. Blood. 1991;78:1373–1380. - PubMed
- Koszinowski U H, Del Val M, Reddehase M. Curr Top Microbiol Immunol. 1990;154:189–220. - PubMed
- Riddell S, Wantanabe K, Goodrich J, Li C R, Agha M, Greenberg P. Science. 1992;257:238–241. - PubMed
- Walter E A, Greenberg P D, Gilberg M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. N Engl J Med. 1995;333:1038–1044. - PubMed
- Beersma M F, Bijlmakers M J, Ploegh H L. J Immunol. 1993;151:4455–4464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous