Brain myosin V is a synaptic vesicle-associated motor protein: evidence for a Ca2+-dependent interaction with the synaptobrevin-synaptophysin complex - PubMed (original) (raw)
Brain myosin V is a synaptic vesicle-associated motor protein: evidence for a Ca2+-dependent interaction with the synaptobrevin-synaptophysin complex
R Prekeris et al. J Cell Biol. 1997.
Abstract
Brain myosin V is a member of a widely distributed class of unconventional myosins that may be of central importance to organelle trafficking in all eukaryotic cells. Molecular constituents that target this molecular motor to organelles have not been previously identified. Using a combination of immunopurification, extraction, cross-linking, and coprecipitation assays, we demonstrate that the tail domain of brain myosin V forms a stable complex with the synaptic vesicle membrane proteins, synaptobrevin II and synaptophysin. While myosin V was principally bound to synaptic vesicles during rest, this putative transport complex was promptly disassembled upon the depolarization-induced entry of Ca2+ into intact nerve endings. Coimmunoprecipitation assays further indicate that Ca2+ disrupts the in vitro binding of synaptobrevin II to synaptophysin in the presence but not in the absence of Mg2+. We conclude that hydrophilic forces reversibly couple the myosin V tail to a biochemically defined class of organelles in brain nerve terminals.
Figures
Figure 1
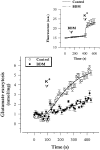
Effect of myosin Mg2+-ATPase inhibition on the Ca2+-dependent release of endogenous glutamate from cerebrocortical synaptosomes. Synaptosomes were preincubated for 5 min in the absence (Control) or presence (BDM) of 10 mM BDM before the onset of membrane depolarization. Ca2+-dependent glutamate release was calculated by subtracting the 45 mM K+-evoked release measured in the presence of 1.3 mM EGTA (Ca2+-independent release) from the release measured in the presence of 1.3 mM CaCl2 (total release). Data are the means ± SEM of duplicate determinations in three independent experiments. Curve fits (solid lines) were obtained using a four-parameter logistic function. (Inset) Changes in synaptosomal membrane potential as measured using the potential-sensitive fluorescent dye DiSC2(5). Synaptosomes were stained with 2 μM DiSC2(5) and fluorescence measurements (expressed in arbitrary units) were made as described in Materials and Methods. BDM (10 mM) and KCl (25 mM) were added at the indicated times (arrowheads). Data are the means ± SEM of five independent determinations.
Figure 2
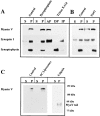
Myosin V copurifies with rab3- and synaptobrevin II (syb)–containing synaptic vesicles. (A) Hippocampal mossy fiber synaptosomes (P3) were isolated from homogenates of hippocampal tissue (Hom.) and subfractioned into a cytosol-enriched fraction (Sol.) and 1% Triton X-100–soluble (TSF) and –insoluble (TIF) fractions. Equal amounts of protein were analyzed for myosins IIA, IIB, and V by immunoblotting. (B) Synaptosomal lysate supernatant (LS1) was incubated with immunobeads coupled to anti-synaptobrevin II mAbs as described in Materials and Methods. The immunopurified synaptic vesicles (SV) were washed in homogenization buffer and analyzed by SDS-PAGE and immunoblotting. Equal volumes of the LS1 and SV fractions were analyzed for myosins IIA, IIB, and V, synapsin I, synaptophysin, and GAP-43. In a separate experiment, rab3- and syb-containing organelles were independently isolated from the LS1 fraction using immunobeads. Sample concentrations were adjusted for loading equal amounts of the vesicle membrane protein synaptophysin and analyzed for the indicated protein by immunoblotting.
Figure 3
Nature of the interaction between myosin V and synaptobrevin II–containing organelles. Immunopurified vesicles were either washed with sucrose-free buffer B (Control) or extracted with 15 mM sodium pyrophosphate (pH 8.2) or 1% Triton X-114 (A); 1 M NaCl (B); and 1 mM phosphatidylcholine (PC) liposomes or exposed to calpain (5 U) (C). Immunobeads were resedimented and the resulting supernatants (S) and pellets (P) were analyzed for myosin V, synapsin I, and synaptophysin by immunoblotting. Immunobead fractions treated with sodium pyrophosphate and probed for synaptophysin were apparently underloaded in the gel shown. Phase separations performed using Triton X-114 yielded an aqueous phase (AP), detergent phase (DP), and insoluble phase (IP). Myo V tail, the ∼80-kD tail fragment generated by calpain cleavage of brain myosin V.
Figure 4
Cross-linking of myosin V to the synaptobrevin II (syb)–synaptophysin (syp) protein complex in intact nerve terminals. (A) Intact cerebrocortical synaptosomes were treated with the chemical cross-linker DTSP and lysed with 1% Triton X-100. Immunoprecipitates were isolated from precleared lysates using antibodies that were raised against synapsin I (synapsin), syp, synaptotagmin I (syt), or syb. Controls were not exposed to primary antibodies before the addition of a secondary IgG (none). DTSP was cleaved by chemical reduction before analyzing each sample by SDS-PAGE and immunoblotting. Two adducts containing myosin V were visible. Anti-syt antibodies nonspecifically reacted with the IgG heavy chains (h.c.). (B) Synaptosomes were incubated in the absence or presence of DTSP, as indicated, before the isolation of a crude synaptic vesicle fraction. Equal amounts of protein were then separated on 5% (top) and 10% (bottom) nonreducing gels, and analyzed by immunoblotting. An adduct of M r >669 kD contained myosin V, syb, and syp.
Figure 5
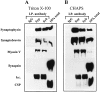
Immunoprecipitation analysis of detergent-solubilized synaptosomes. Triton X-100– (A) and CHAPS-solubilized (B) samples were subjected to immunoprecipitation (I.P.) as described in Materials and Methods, with mAbs against synaptophysin (Syp) or synaptobrevin (Syb). Control samples were incubated with the secondary IgG alone (A and B; IgG). Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting for the presence of Syp, Syb II, myosin V, synapsin I, and cysteine string protein (CSP). A sample corresponding to 10% of the total material used for all immunoprecipitations is shown in the right column of each panel (10% total).
Figure 6
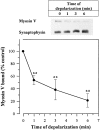
Depolarization dissociated the myosin V–vesicle complex in intact nerve terminals. Intact cerebrocortical synaptosomes were incubated for 6 min in the presence of 1.4 mM CaCl2. The concentration of KCl was increased to 40 mM at 0, 1, 3, and 6 min of incubation, giving the specified time of depolarization. Crude synaptic vesicle fractions were immediately isolated and analyzed for myosin V and synaptophysin by SDS-PAGE and immunoblotting. Equal amounts of protein were loaded onto each gel. Synaptophysin was used as a control to correct for the corresponding amounts of myosin V in each sample and the nondepolarized (0 min) values were normalized to 100%. Data are the means ± SEM of four independent experiments.
Figure 7
Ca2+ cooperates with Mg2+ to reverse the binding of myosin V to synaptic vesicles. (A) Synaptic vesicles were immunoisolated from synaptosomal lysates in the presence of 2 mM EGTA or the specified divalent ion(s). All ions were used at a final concentration of 100 μM, except Mg2+ (2.5 mM) and Pb2+(0.1 μM). The concentration of Pb2+ used in this study corresponds to the value that produces maximal secretory activity (unpublished observation). (B) Synaptic vesicles were immunoisolated from synaptosomal lysates in the presence of 2.5 mM Mg2+and either 2 mM EGTA or 100 μM Ca2+, Ba2+, Sr2+, or 0.1 μM Pb2+. (C) Synaptic vesicles were immunoisolated from synaptosomal lysates in the presence of 2.5 mM Mg2+ and the specified concentrations of Ca2+. Coimmunopurified proteins were separated by SDS-PAGE. Synaptophysin was used to control for equal loading of all samples and changes in the amount of vesicle-bound myosin V were analyzed by immunoblotting.
Figure 8
Ca2+ cooperates with Mg2+ to reverse synaptobrevin II binding to synaptophysin in an ATP-dependent manner. (A) Rat cerebrocortical synaptosomal lysates were incubated for 10 min at 37°C in the presence of 1 mM ATP and either excess chelator (2 mM_EGTA_) or 100 μM CaCl2 in the absence (Ca2 +) or presence (Mg–Ca) of 2.5 mM MgCl2. Triton X-100 (1% vol/vol) extracts were precleared with protein A–agarose as described under Materials and Methods and subjected to immunoprecipitation using mAbs directed against synaptobrevin II (syb) or synaptophysin (syp). Immunoprecipitates were washed and analyzed by SDS-PAGE and immunoblotting using both the anti-syb and anti-syp antibodies. Samples were loaded so that the intensities of syb and syp bands were equivalent for each experimental treatment when samples were immunoprecipitated and immunoblotted using the same antibodies. (B) Immunoprecipitations from cerebrocortical lysates were performed according to the procedure described above (A) in the absence or presence of 1 mM ATP or ATPγS, as indicated.
Similar articles
- The synaptophysin-synaptobrevin complex is developmentally upregulated in cultivated neurons but is absent in neuroendocrine cells.
Becher A, Drenckhahn A, Pahner I, Ahnert-Hilger G. Becher A, et al. Eur J Cell Biol. 1999 Sep;78(9):650-6. doi: 10.1016/S0171-9335(99)80050-8. Eur J Cell Biol. 1999. PMID: 10535307 - Globular tail of myosin-V is bound to vamp/synaptobrevin.
Ohyama A, Komiya Y, Igarashi M. Ohyama A, et al. Biochem Biophys Res Commun. 2001 Feb 2;280(4):988-91. doi: 10.1006/bbrc.2001.4236. Biochem Biophys Res Commun. 2001. PMID: 11162623 - The C-terminal transmembrane region of synaptobrevin binds synaptophysin from adult synaptic vesicles.
Yelamanchili SV, Reisinger C, Becher A, Sikorra S, Bigalke H, Binz T, Ahnert-Hilger G. Yelamanchili SV, et al. Eur J Cell Biol. 2005 Apr;84(4):467-75. doi: 10.1016/j.ejcb.2004.11.007. Eur J Cell Biol. 2005. PMID: 15900706 - Myosin-V: a class of unconventional molecular motors.
Larson RE. Larson RE. Braz J Med Biol Res. 1996 Mar;29(3):309-18. Braz J Med Biol Res. 1996. PMID: 8736123 Review. - The Sybtraps: control of synaptobrevin traffic by synaptophysin, α-synuclein and AP-180.
Gordon SL, Cousin MA. Gordon SL, et al. Traffic. 2014 Mar;15(3):245-54. doi: 10.1111/tra.12140. Epub 2013 Dec 16. Traffic. 2014. PMID: 24279465 Free PMC article. Review.
Cited by
- Role of myosin Va in purinergic vesicular neurotransmission in the gut.
Chaudhury A, He XD, Goyal RK. Chaudhury A, et al. Am J Physiol Gastrointest Liver Physiol. 2012 Mar 15;302(6):G598-607. doi: 10.1152/ajpgi.00330.2011. Epub 2011 Dec 29. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22207579 Free PMC article. - The micro-architecture of mitochondria at active zones: electron tomography reveals novel anchoring scaffolds and cristae structured for high-rate metabolism.
Perkins GA, Tjong J, Brown JM, Poquiz PH, Scott RT, Kolson DR, Ellisman MH, Spirou GA. Perkins GA, et al. J Neurosci. 2010 Jan 20;30(3):1015-26. doi: 10.1523/JNEUROSCI.1517-09.2010. J Neurosci. 2010. PMID: 20089910 Free PMC article. - The synaptic vesicle protein synaptophysin: purification and characterization of its channel activity.
Gincel D, Shoshan-Barmatz V. Gincel D, et al. Biophys J. 2002 Dec;83(6):3223-9. doi: 10.1016/S0006-3495(02)75324-1. Biophys J. 2002. PMID: 12496091 Free PMC article. - Functional Role of Gonadotrope Plasticity and Network Organization.
Edwards BS, Clay CM, Ellsworth BS, Navratil AM. Edwards BS, et al. Front Endocrinol (Lausanne). 2017 Sep 7;8:223. doi: 10.3389/fendo.2017.00223. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28936197 Free PMC article. Review. - Recently recycled synaptic vesicles use multi-cytoskeletal transport and differential presynaptic capture probability to establish a retrograde net flux during ISVE in central neurons.
Parkes M, Landers NL, Gramlich MW. Parkes M, et al. Front Cell Dev Biol. 2023 Nov 6;11:1286915. doi: 10.3389/fcell.2023.1286915. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38020880 Free PMC article.
References
- Bernstein BW, Bamburg JR. Cycling of actin assembly in synaptosomes and neurotransmitter release. Neuron. 1989;3:257–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous