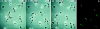Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis - PubMed (original) (raw)
Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis
M Nieto et al. J Exp Med. 1997.
Abstract
Leukocyte migration in response to cell attractant gradients or chemotaxis is a key phenomenon both in cell movement and in the inflammatory response. Chemokines are quite likely to be the key molecules directing migration of leukocytes that involve cell polarization with generation of specialized cell compartments. The precise mechanism of leukocyte chemoattraction is not known, however. In this study, we demonstrate that the CC chemokine receptors CCR2 and CCR5, but not cytokine receptors such as interleukin (IL)-2Ralpha, IL-2Rbeta, tumor necrosis factor receptor 1, or transforming growth factor betaR, are redistributed to a pole in T cells that are migrating in response to chemokines. Immunofluorescence and confocal microscopy studies show that the chemokine receptors concentrate at the leading edge of the cell on the flattened cell-substratum contact area, induced specifically by the signals that trigger cell polarization. The redistribution of chemokine receptors is blocked by pertussis toxin and is dependent on cell adhesion through integrin receptors, which mediate cell migration. Chemokine receptor expression on the leading edge of migrating polarized lymphocytes appears to act as a sensor mechanism for the directed migration of leukocytes through a chemoattractant gradient.
Figures
Figure 1
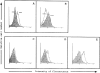
Recognition of human CCR2 and CCR5 chemokine receptors in transfected 293 cells and PBL. Flow cytometry analysis of the binding of the anti-CCR2 mAb MCP-1R03 (A) and mouse anti-CCR5 antibody (B) to the 293 cell line, transiently transfected with human CCR2 (white histogram) and CCR5 (shaded histogram) cDNA. CCR2 expression was assessed using the anti-CCR2 MCP-1R03 mAb in resting (C) or PHA-activated IL-2–cultured human PBL (D). Expression of the CCR5 in activated human PBL is shown (E). Shaded histograms correspond to the isotype-matched mAb (C and D) and irrelevant mouse antiserum (E) negative-staining controls. Cells were incubated with biotinylated anti-CCR2 mAb MCP-1R03 or mouse anti-CCR5 antiserum for 30 min at 4°C. Cell-bound fluorescence was determined in a flow cytometer (Profile XL; Coulter Corp.).
Figure 2
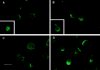
The CCR2 and the CCR5 receptors redistribute to the leading edge of the polarized migrating T cells. PHA-activated T lymphocytes, untreated (A and C), or treated with 10 ng/ml MCP-1 (B) or 10 ng/ml RANTES (D), were allowed to adhere to coverslips coated with 20 μg/ml FN-80 (A and B) or 10 μg/ml rsICAM-1-Fc (C and D), and then fixed and stained with the anti-CCR2 mAb MCP-1R03 (A and B) or the anti-CCR5 mouse antiserum (C and D), as described in Materials and Methods. Cells were photographed under epifluorescent conditions. Original magnification: ×1,200. Bar, 10 μm. Insets show staining of the IL-2Rα with the TP1/6 mAb on a resting (A) and a polarized (B) T lymphoblast. Original magnification: ×600.
Figure 3
Migrating T cells polarize the CCR2 receptor towards the chemoattractant gradient. (A–C) Time-lapse videomicroscopy analysis of migrating T lymphoblasts in response to MCP-1. Cells were allowed to adhere to 10 μg/ml rsICAM-1 on coverslips; MCP-1 was then deposited at one edge of the bottom of the well and cells allowed to migrate. Sequential time frames are shown. Polarized migrating lymphocytes show the phase-dark leading edge and the phase-bright uropod. (D) Parallel immunofluorescence studies show the CCR2 distribution of the migrating cells. Cells were photographed with a ×60 objective. Bar, 10 μm. The arrows indicate the direction of cell migration towards the chemokine source.
Figure 4
(a) The CCR2 receptor patches on the cell-sustratum contact areas at the leading edge of the migrating T cell. Confocal microscopy was performed as described in Materials and Methods. Three representative optical cell sections are shown, out of the eight obtained. Serial sections are from the upper level (left, 2.464 μm), which shows staining of ICAM-3 (red fluorescence) at the uropod, to the sustratum level (right, 3.92 μm), where CCR-2 (green fluorescence) is found at the leading edge. (b) Polarized distribution of the CCR2 receptor and ICAM-3 on migrating T cells. Cells adhered to FN-80–coated coverslips were double stained with anti-ICAM-3 HP2/19 (yellow fluorescence) and anti-CCR2 mAb MCP-1R03 (green fluorescence). Original magnification: ×1,200. Bar, 10 μm. The inset shows another T lymphoblast from the same sample at equal magnification.
Figure 4
(a) The CCR2 receptor patches on the cell-sustratum contact areas at the leading edge of the migrating T cell. Confocal microscopy was performed as described in Materials and Methods. Three representative optical cell sections are shown, out of the eight obtained. Serial sections are from the upper level (left, 2.464 μm), which shows staining of ICAM-3 (red fluorescence) at the uropod, to the sustratum level (right, 3.92 μm), where CCR-2 (green fluorescence) is found at the leading edge. (b) Polarized distribution of the CCR2 receptor and ICAM-3 on migrating T cells. Cells adhered to FN-80–coated coverslips were double stained with anti-ICAM-3 HP2/19 (yellow fluorescence) and anti-CCR2 mAb MCP-1R03 (green fluorescence). Original magnification: ×1,200. Bar, 10 μm. The inset shows another T lymphoblast from the same sample at equal magnification.
Figure 5
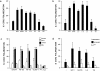
(a and b) The role of chemoattractants in the redistribution of CCR2 and CCR5 receptors on migrating T lymphocytes. T lymphoblasts stimulated with 10 ng/ml of several polarizing chemokines and cytokines were allowed to adhere to coverslips coated with 20 μg/ml FN-80 (a) or 10 μg/ml rsICAM-1-Fc (b). Cells were then stained and the percentage of cells on which the CCR2 (a) or CCR5 (b) receptors were redistributed was calculated as described. The arithmetic mean ± SEM of four (a) and three (b) independent experiments is shown. (c) The role of various substrates on CCR2 receptor polarization. T lymphoblasts were allowed to adhere to coverslips coated with 10 μg/ml rsICAM-1-Fc, 20 μg/ml FN-80, 30 μg/ml FN-40, 20 μg/ml rsVCAM-1-Fc, or 20 μg/ml poly-L-Lys, and then stimulated with chemokines or PMA (10 ng/ml). Cell samples were processed as described. The arithmetic mean ± SEM of three independent experiments is represented. (d) Chemokine-induced polarization of the CCR2 receptor is blocked by pertussis toxin. PHA-activated T lymphocytes were pretreated with pertussis toxin (1 μg/ml) for 20 min at 37°C, and then allowed to adhere to rsICAM-1 (as in the legend to Fig. 2). The effect of the chemokines on CCR2 redistribution to the leading edge of cells was quantified as in Fig. 2. The arithmetic mean ± SEM of three independent experiments is shown.
Similar articles
- Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes.
Loetscher P, Seitz M, Baggiolini M, Moser B. Loetscher P, et al. J Exp Med. 1996 Aug 1;184(2):569-77. doi: 10.1084/jem.184.2.569. J Exp Med. 1996. PMID: 8760810 Free PMC article. - Differential expression of chemokines and chemokine receptors in inflammatory periapical diseases.
Silva TA, Garlet GP, Lara VS, Martins W Jr, Silva JS, Cunha FQ. Silva TA, et al. Oral Microbiol Immunol. 2005 Oct;20(5):310-6. doi: 10.1111/j.1399-302X.2005.00232.x. Oral Microbiol Immunol. 2005. PMID: 16101967 - The two poles of the lymphocyte: specialized cell compartments for migration and recruitment.
del Pozo MA, Nieto M, Serrador JM, Sancho D, Vicente-Manzanares M, Martínez C, Sánchez-Madrid F. del Pozo MA, et al. Cell Adhes Commun. 1998;6(2-3):125-33. doi: 10.3109/15419069809004468. Cell Adhes Commun. 1998. PMID: 9823463 Review. - Eotaxin activates T cells to chemotaxis and adhesion only if induced to express CCR3 by IL-2 together with IL-4.
Jinquan T, Quan S, Feili G, Larsen CG, Thestrup-Pedersen K. Jinquan T, et al. J Immunol. 1999 Apr 1;162(7):4285-92. J Immunol. 1999. PMID: 10201960 - Chemokines as regulators of T cell differentiation.
Luther SA, Cyster JG. Luther SA, et al. Nat Immunol. 2001 Feb;2(2):102-7. doi: 10.1038/84205. Nat Immunol. 2001. PMID: 11175801 Review.
Cited by
- CCR2 acts as scavenger for CCL2 during monocyte chemotaxis.
Volpe S, Cameroni E, Moepps B, Thelen S, Apuzzo T, Thelen M. Volpe S, et al. PLoS One. 2012;7(5):e37208. doi: 10.1371/journal.pone.0037208. Epub 2012 May 17. PLoS One. 2012. PMID: 22615942 Free PMC article. - Polarization of migrating monocytic cells is independent of PI 3-kinase activity.
Volpe S, Thelen S, Pertel T, Lohse MJ, Thelen M. Volpe S, et al. PLoS One. 2010 Apr 15;5(4):e10159. doi: 10.1371/journal.pone.0010159. PLoS One. 2010. PMID: 20419163 Free PMC article. - Altered CXCR4 dynamics at the cell membrane impairs directed cell migration in WHIM syndrome patients.
García-Cuesta EM, Rodríguez-Frade JM, Gardeta SR, D'Agostino G, Martínez P, Soler Palacios B, Cascio G, Wolf T, Mateos N, Ayala-Bueno R, Santiago CA, Lucas P, Llorente L, Allende LM, González-Granado LI, Martín-Cófreces N, Roda-Navarro P, Sallusto F, Sánchez-Madrid F, García-Parajo MF, Martínez-Muñoz L, Mellado M. García-Cuesta EM, et al. Proc Natl Acad Sci U S A. 2022 May 24;119(21):e2119483119. doi: 10.1073/pnas.2119483119. Epub 2022 May 19. Proc Natl Acad Sci U S A. 2022. PMID: 35588454 Free PMC article. - Role of Hydrogen Sulfide, Substance P and Adhesion Molecules in Acute Pancreatitis.
Kumar A, Bhatia M. Kumar A, et al. Int J Mol Sci. 2021 Nov 9;22(22):12136. doi: 10.3390/ijms222212136. Int J Mol Sci. 2021. PMID: 34830018 Free PMC article. Review. - How human leukocytes track down and destroy pathogens: lessons learned from the model organism Dictyostelium discoideum.
Jin T, Xu X, Fang J, Isik N, Yan J, Brzostowski JA, Hereld D. Jin T, et al. Immunol Res. 2009;43(1-3):118-27. doi: 10.1007/s12026-008-8056-7. Immunol Res. 2009. PMID: 18827980 Review.
References
- Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. - PubMed
- Quin S, LaRosa G, Campbell JJ, Smith-Heath H, Kassam N, Shi X, Zeng L, Butcher EC, Mackay CR. Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: correlation with transendothelial chemotactic potential. Eur J Immunol. 1996;26:640–647. - PubMed
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources