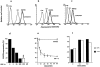HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication - PubMed (original) (raw)
HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication
A Amara et al. J Exp Med. 1997.
Abstract
Ligation of CCR5 by the CC chemokines RANTES, MIP-1alpha or MIP-1beta, and of CXCR4 by the CXC chemokine SDF-1alpha, profoundly inhibits the replication of HIV strains that use these coreceptors for entry into CD4(+) T lymphocytes. The mechanism of entry inhibition is not known. We found a rapid and extensive downregulation of CXCR4 by SDF-1alpha and of CCR5 by RANTES or the antagonist RANTES(9-68). Confocal laser scanning microscopy showed that CCR5 and CXCR4, after binding to their ligands, are internalized into vesicles that qualify as early endosomes as indicated by colocalization with transferrin receptors. Internalization was not affected by treatment with Bordetella pertussis toxin, showing that it is independent of signaling via Gi-proteins. Removal of SDF-1alpha led to rapid, but incomplete surface reexpression of CXCR4, a process that was not inhibited by cycloheximide, suggesting that the coreceptor is recycling from the internalization pool. Deletion of the COOH-terminal, cytoplasmic domain of CXCR4 did not affect HIV entry, but prevented SDF-1alpha-induced receptor downregulation and decreased the potency of SDF-1alpha as inhibitor of HIV replication. Our results indicate that the ability of the coreceptor to internalize is not required for HIV entry, but contributes to the HIV suppressive effect of CXC and CC chemokines.
Figures
Figure 1
Effect of SDF-1α stimulation on CXCR4 surface expression. CEM lymphoblastoid T cells (a), monocyte-depleted PBMC (b), or HeLa cells (c) were treated for 40 min at 37°C with 200 nM SDF-1α, 200 nM RANTES, or medium alone (UNTREATED). The cells were then washed with acidic glycine buffer, labeled at 4°C with anti-CXCR4 and a PE-conjugated secondary antibody, and analyzed by flow cytometry. In b, analysis was performed on gated CD4+ cells (FITC-conjugated anti-CD4). Control cells (CTRL) were labeled with the secondary antibody only. (d) Dependence of CXCR4 downregulation on SDF-1α concentration. CEM cells were incubated for 40 min at 37°C with increasing concentrations of SDF-1α, and surface expression of CXCR4 was determined. PTX: before incubation with SDF-1α, the cells were treated with 5 μg/ml pertussis toxin for 90 min. (e) Time course of CXCR4 downregulation. Cells were pre-incubated at 4°C for 60 min with 200 nM SDF-1α or RANTES. After washing, cells were cultured at 37°C for the indicated times in the absence of chemokines. ( f ) Re-expression of CXCR4. The cells were incubated at 37°C for 40 min with 200 nM SDF-1α in the presence or absence of 100 μg/ml cycloheximide, washed in acidic glycine buffer and further cultured for up to 60 min at 37 °C with or without cycloheximide in the absence of SDF-1α. (d–f ) Relative surface expression of CXCR4 was analyzed by flow cytometry as described for a.
Figure 2
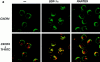
Internalization of CXCR4 (a) and CCR5 (b and c) were analyzed by confocal laser scanning microscopy in CEM (a), CHO-CCR5-GFP (b), and HeLa-CCR5-GFP (c) cells after exposure to 200 nM SDF-1α, RANTES or RANTES(9-68) for 30 min at 37°C. –, untreated cells; CXCR4, cells labeled with anti-CXCR4 and a FITC-conjugated secondary antibody; CCR5, autofluorescence of CCR5-GFP; CXCR4+, Tf-RITC and CCR5+Tf-RITC, simultaneous detection of Tf-RITC (red ) and either CXCR4 or CCR5-GFP ( green). Yellow spots indicate colocalization of chemokine receptor and Tf-RITC.
Figure 2
Internalization of CXCR4 (a) and CCR5 (b and c) were analyzed by confocal laser scanning microscopy in CEM (a), CHO-CCR5-GFP (b), and HeLa-CCR5-GFP (c) cells after exposure to 200 nM SDF-1α, RANTES or RANTES(9-68) for 30 min at 37°C. –, untreated cells; CXCR4, cells labeled with anti-CXCR4 and a FITC-conjugated secondary antibody; CCR5, autofluorescence of CCR5-GFP; CXCR4+, Tf-RITC and CCR5+Tf-RITC, simultaneous detection of Tf-RITC (red ) and either CXCR4 or CCR5-GFP ( green). Yellow spots indicate colocalization of chemokine receptor and Tf-RITC.
Figure 2
Internalization of CXCR4 (a) and CCR5 (b and c) were analyzed by confocal laser scanning microscopy in CEM (a), CHO-CCR5-GFP (b), and HeLa-CCR5-GFP (c) cells after exposure to 200 nM SDF-1α, RANTES or RANTES(9-68) for 30 min at 37°C. –, untreated cells; CXCR4, cells labeled with anti-CXCR4 and a FITC-conjugated secondary antibody; CCR5, autofluorescence of CCR5-GFP; CXCR4+, Tf-RITC and CCR5+Tf-RITC, simultaneous detection of Tf-RITC (red ) and either CXCR4 or CCR5-GFP ( green). Yellow spots indicate colocalization of chemokine receptor and Tf-RITC.
Figure 3
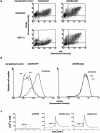
Effect of deletion of the COOH-terminal cytoplasmic domain of CXCR4. (a) HeLa cells were transiently transfected with the CXCR4 WT or the CXCR4 ΔCyt expression vector, along with a plasmid encoding the reporter gene GFP (pEGFP). 24 h later, the cells were incubated for 45 min at 37°C in the presence or absence of 300 nM SDF-1α, labeled with anti-CXCR4, and analyzed by flow cytometry. Expression of GFP allows to distinguish transfected (GFP +) and nontransfected (GFP −) cells. After transfection with CXCR4 WT or CXCR4 Δcyt, SDF-1α–dependent downregulation of the endogenous and the transfected receptor were monitored in GFP− and GFP+ cells, respectively. (–) HeLa cells were transiently transfected with the CXCR4 WT or CXCR4 ΔCyt expression vector, along with the pEGFP plasmid. 24 h later, the cells were incubated for 45 min at 37°C with 300 nM SDF-1α or with 20 ng/ml PMA, labeled with anti-CXCR4 and surface expression of CXCR4 was analyzed by flow cytometry in GFP+ cells. (c) CHO cells were transfected with either the CXCR4 WT or the CXCR4 ΔCyt expression vectors and were loaded 48 h later with Fura-2. CHO control cells were transfected with vector DNA alone (pcDNA3). Recordings of [Ca2+]i changes after stimulation with 200 nM of SDF-1α are shown.
Figure 4
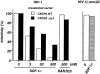
SDF-1α–dependent inhibition of HIV infection in cells expressing wild-type or COOH-terminally truncated CXCR4. U373-CD4 LTRlacZ were transfected with either CXCR4 WT or CXCR4 ΔCyt expression vector, plated in 96-well plates (104 cells per well) and infected with the HIV-1NL4-3 strain or the pseudotyped HIV-1(Δenv)G, in the presence of the indicated concentrations of SDF-1α or RANTES. HIV-1(Δenv)G–infected cells were treated with 500 nM SDF-1α. Cell cultures were carried out in triplicates. Surface expression of CXCR4 WT or CXCR4 ΔCyt was assessed 24 h after transfection by FACS® analysis and amounted to 482 and 533 fluorescence units, respectively. HIV infection was revealed 24 h later by staining for β-galactosidase activity and scoring of positive cells. The numbers of HIV-infected cells per well in the absence of chemokines were 168 and 232 (mean of five experiments) for cultures transfected with CXCR4 WT and CXCR4 ΔCyt, respectively. In cultures infected with HIV-1(Δ_env_)G the average number of infected cells per well was 280. Shown are the percentages of infected cells in the presence of chemokines. Infection with HIV-1NL4-3 or HIV-1(Δenv)G in the absence of chemokines was set to 100%.
Similar articles
- Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1.
Xiang J, George SL, Wünschmann S, Chang Q, Klinzman D, Stapleton JT. Xiang J, et al. Lancet. 2004 Jun 19;363(9426):2040-6. doi: 10.1016/S0140-6736(04)16453-2. Lancet. 2004. PMID: 15207954 - Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4.
Signoret N, Oldridge J, Pelchen-Matthews A, Klasse PJ, Tran T, Brass LF, Rosenkilde MM, Schwartz TW, Holmes W, Dallas W, Luther MA, Wells TN, Hoxie JA, Marsh M. Signoret N, et al. J Cell Biol. 1997 Nov 3;139(3):651-64. doi: 10.1083/jcb.139.3.651. J Cell Biol. 1997. PMID: 9348282 Free PMC article. - In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression.
Scarlatti G, Tresoldi E, Björndal A, Fredriksson R, Colognesi C, Deng HK, Malnati MS, Plebani A, Siccardi AG, Littman DR, Fenyö EM, Lusso P. Scarlatti G, et al. Nat Med. 1997 Nov;3(11):1259-65. doi: 10.1038/nm1197-1259. Nat Med. 1997. PMID: 9359702 - HIV co-receptors as targets for antiviral therapy.
Schols D. Schols D. Curr Top Med Chem. 2004;4(9):883-93. doi: 10.2174/1568026043388501. Curr Top Med Chem. 2004. PMID: 15134547 Review. - [Role of chemokines in the HIV infection process].
Proost P, Schols D. Proost P, et al. Verh K Acad Geneeskd Belg. 2002;64(6):403-20. Verh K Acad Geneeskd Belg. 2002. PMID: 12649932 Review. Dutch.
Cited by
- Bacterial vaginosis-driven changes in cervicovaginal immunity that expand the immunological hypothesis for increased HIV susceptibility.
MacLean F, Tsegaye AT, Graham JB, Swarts JL, Vick SC, Potchen N, Talavera IC, Warrier L, Dubrulle J, Schroeder LK, Saito A, Thomas KK, Mack M, Sabo MC, Chohan BH, Ngure K, Mugo N, Lingappa JR, Lund JM. MacLean F, et al. bioRxiv [Preprint]. 2025 Jan 14:2024.07.03.601916. doi: 10.1101/2024.07.03.601916. bioRxiv. 2025. PMID: 39005354 Free PMC article. Preprint. - Inhibitory mechanism of the CXCR4 antagonist T22 against human immunodeficiency virus type 1 infection.
Murakami T, Zhang TY, Koyanagi Y, Tanaka Y, Kim J, Suzuki Y, Minoguchi S, Tamamura H, Waki M, Matsumoto A, Fujii N, Shida H, Hoxie JA, Peiper SC, Yamamoto N. Murakami T, et al. J Virol. 1999 Sep;73(9):7489-96. doi: 10.1128/JVI.73.9.7489-7496.1999. J Virol. 1999. PMID: 10438838 Free PMC article. - Interaction of the CC-chemokine RANTES with glycosaminoglycans activates a p44/p42 mitogen-activated protein kinase-dependent signaling pathway and enhances human immunodeficiency virus type 1 infectivity.
Chang TL, Gordon CJ, Roscic-Mrkic B, Power C, Proudfoot AE, Moore JP, Trkola A. Chang TL, et al. J Virol. 2002 Mar;76(5):2245-54. doi: 10.1128/jvi.76.5.2245-2254.2002. J Virol. 2002. PMID: 11836402 Free PMC article. - Disturbed CD4+ T cell homeostasis and in vitro HIV-1 susceptibility in transgenic mice expressing T cell line-tropic HIV-1 receptors.
Sawada S, Gowrishankar K, Kitamura R, Suzuki M, Suzuki G, Tahara S, Koito A. Sawada S, et al. J Exp Med. 1998 May 4;187(9):1439-49. doi: 10.1084/jem.187.9.1439. J Exp Med. 1998. PMID: 9565636 Free PMC article. - Endocytic Trafficking of HIV gp120 is Mediated by Dynamin and Plays a Role in gp120 Neurotoxicity.
Wenzel ED, Bachis A, Avdoshina V, Taraballi F, Tasciotti E, Mocchetti I. Wenzel ED, et al. J Neuroimmune Pharmacol. 2017 Sep;12(3):492-503. doi: 10.1007/s11481-017-9739-4. Epub 2017 Mar 27. J Neuroimmune Pharmacol. 2017. PMID: 28349243 Free PMC article.
References
- Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. - PubMed
- Broder CC, Dimitrov DS, Blumenthal R, Berger EA. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s) Virology. 1993;193:483–491. - PubMed
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science (Wash DC) 1996;272:872–877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials