BRCA1 is a cell cycle-regulated nuclear phosphoprotein - PubMed (original) (raw)
BRCA1 is a cell cycle-regulated nuclear phosphoprotein
H Ruffner et al. Proc Natl Acad Sci U S A. 1997.
Abstract
We have characterized the BRCA1 gene product by using four polyclonal antibodies raised against peptides from four different regions of the protein. The antibodies specifically recognize an approximately 220-kDa BRCA1 protein that is predominantly expressed in the nucleus of both normal and neoplastic breast cancer cells. It is a serine phosphoprotein that undergoes hyperphosphorylation during late G1 and S phases of the cell cycle and is transiently dephosphorylated early after M phase. We propose that BRCA1 is a phosphoprotein that alters in a qualitative and quantitative manner during cell cycle progression.
Figures
Figure 1
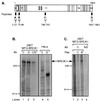
Characterization of BRCA1 antibodies. (A) Diagram showing BRCA1 epitopes against which antibodies were raised. The horizontal bar represents BRCA1 protein consisting of amino acids 1–1863; vertical lines indicate borders of coding exons, some of which are numbered. Open box, RING domain; hatched boxes, putative nuclear localization signals. Antibodies were raised against four different synthetic BRCA1 peptides that were derived from the BRCA1 protein sequence, as indicated by the black boxes (designated A–D). The corresponding amino acid numbers are indicated below. (B) Antibodies Ab-C and Ab-D immunoprecipitate in vivo overexpressed and endogenous BRCA1. The lysates from [35S]methionine-labeled 293T cells transfected with pCL-MFG-BRCA1 (MFG-BRCA1; lanes 1–3) or untransfected HeLa cells (lanes 4 and 5) were immunoprecipitated by the following antibodies: Ab-C, lanes 1 and 4; Ab-D, lanes 2 and 5; Ab-C and -D consecutively, lane 3. (C) BRCA1 protein was precipitated by Ab-C from overexpressing [35S]methionine-labeled 293T cells in the absence or presence of competitor peptide. Lane 1, no peptide added; lane 2, corresponding peptide Pep-C added; lane 3, noncorresponding peptide Pep-B added; lane 4, normal rabbit serum (NS) used for precipitation, no peptide added. Arrows indicate BRCA1 protein. Protein marker bands (200 and 97.4 kDa) are marked on the side.
Figure 2
In vitro translated, immunoprecipitated endogenous, and overexpressed BRCA1 reveal the same tryptic peptide pattern. Shown are tryptic peptide maps of overexpressed BRCA1 in 293T cells (Left), endogenous BRCA1 from HeLa cells (Center), and in vitro translated BRCA1 (Right). Immunoprecipitations were performed using either Ab-C or Ab-D, and the precipitates were separated by SDS/PAGE (see Fig. 1_B_, lanes 1 and 2 and 4 and 5, respectively). BRCA1 from Ab-C and Ab-D precipitations was pooled for each cell type. Tryptic peptide analysis was performed on each pool. The diamonds mark sample origins. Circles (numbered 1–11) indicate common spots in the three maps. Comparable maps were also obtained for endogenous BRCA1 from pooled immunoprecipitates from 293T cells and for overexpressed BRCA1 from 293T cells analyzed after precipitation by either Ab-C or -D alone (data not shown).
Figure 3
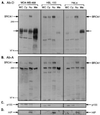
Subcellular localization of BRCA1. Human breast cancer cells (MDA-MB-468), “normal” breast cells (HBL-100), and cervical cancer cells (HeLa) were separated into cytoplasmic (Cy), nuclear (Nu), and membrane (Me) fractions. WC, whole cell extracts. Proteins were separated by SDS/PAGE and blotted to Immobilon-P membranes. Equal amounts of protein per cell line were loaded in each lane. Ab-D (A) and Ab-A (B) were used as primary antibodies for hybridizations. The filled arrows indicate BRCA1 protein. The open arrows indicate the position of the ≈150-kDa crossreacting protein(s). To inspect the fractionation procedure, immunoblotting was performed by using an antibody against NFκB p52 (Santa Cruz Biotechnology) detecting p100 (C) and by an antibody against HIP116 (D).
Figure 4
BRCA1 is a phosphoprotein. (A) Immunoprecipitation of overexpressed and endogenous BRCA1 from cells labeled with [32P]phosphoric acid. BRCA1 was precipitated from lysates of HeLa cells (lane 1) and overexpressing 293T cells (lane 2) by using Ab-C. For comparison, overexpressing 293T cells were labeled with [35S]methionine, and BRCA1 was precipitated by Ab-C (lane 3) or preimmune serum (PS, lane 4). (B) Phosphoamino acid analyses of BRCA1 protein labeled in vivo with [32P]phosphoric acid. BRCA1 was precipitated from the following cells: a, 293T cells overexpressing BRCA1; b, mock-transfected 293T cells; c, HeLa cells; d, HBL-100 cells [from nuclear extract; a similar result was obtained for BRCA1 precipitated from cytoplasmic extract (data not shown)]. Pi, orthophosphate; S, T, and Y, phosphoserine, phosphothreonine, and phosphotyrosine, respectively. Electrophoresis was performed in two dimensions, as indicated by the arrows. Diamonds, sample origins. (C) Tryptic phosphopeptide map of in vivo overexpressed BRCA1. A tryptic digestion was performed on BRCA1 precipitated from the lysate of overexpressing, [32P]phosphoric acid-labeled 293T cells. The resulting peptides were separated in the first and second dimension by electrophoresis and chromatography, respectively, as indicated by the arrows. Peptides p14–p16 probably represent partially digested fragments.
Figure 5
Cell cycle-specific changes of BRCA1. (A and B) Change of BRCA1 protein in HeLa cells following release of a nocodazole-induced M-phase block (A) and a thymidine–aphidicolin G1/S block (B). (C) HeLa cells were separated by centrifugal elutriation according to size. (Upper) Immunoblotting of proteins from cell lysates by Ab-D. Numbers above the panels denote hours after block release (A and B) or individual elutriated fractions (C). The bands representing BRCA1 protein (filled arrows) and the crossreacting ≈150-kDa species (open arrows) are indicated on the side. (Lower) Corresponding cellular DNA contents. The numbers in the tables represent the number of cells (in percentage) in G0/G1, S, and G2/M at different times after block release. *Due to limited number of cells, only 2,784 cells were analyzed by FACS for DNA content.
Figure 6
BRCA1 phosphorylation is increased in S phase. HeLa cells were released from a nocodazole-induced block as described for Fig. 5. (Upper) (A) Immunoblotting by Ab-D for BRCA1 from lysates of cells that were released from the block for different periods of time. (B) BRCA1 was immunoprecipitated from the same lysates as described for A by Ab-D, prior to incubation without (−) or with (+) BAP. The precipitates were separated by SDS/PAGE and immunoblotted using Ab-D. Numbers above the panels indicate hours after block release. (Lower) Cellular DNA content after each time period.
References
- Hall J M, Lee M K, Newman B, Morrow J E, Anderson L A, Huey B, King M-C. Science. 1990;250:1684–1689. - PubMed
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal P A, Harshman K, et al. Science. 1994;266:66–71. - PubMed
- Castilla L H, Couch F J, Erdos M R, Hoskins K F, Calzone K, Garber J E, Boyd J, Lubin M B, Deshano M L, Brody L C, Collins F S, Weber B L. Nat Genet. 1994;8:387–391. - PubMed
- Futreal P A, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, et al. Science. 1994;266:120–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous