Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease - PubMed (original) (raw)
Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease
S Hunot et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Evidence from postmortem studies suggest an involvement of oxidative stress in the degeneration of dopaminergic neurons in Parkinson disease (PD) that have recently been shown to die by apoptosis, but the relationship between oxidative stress and apoptosis has not yet been elucidated. Activation of the transcription factor NF-kappaB is associated with oxidative stress-induced apoptosis in several nonneuronal in vitro models. To investigate whether it may play a role in PD, we looked for the translocation of NF-kappaB from the cytoplasm to the nucleus, evidence of its activation, in melanized neurons in the mesencephalon of postmortem human brain from five patients with idiopathic PD and seven matched control subjects. In PD patients, the proportion of dopaminergic neurons with immunoreactive NF-kappaB in their nuclei was more than 70-fold that in control subjects. A possible relationship between the nuclear localization of NF-kappaB in mesencephalic neurons of PD patients and oxidative stress in such neurons has been shown in vitro with primary cultures of rat mesencephalon, where translocation of NF-kappaB is preceded by a transient production of free radicals during apoptosis induced by activation of the sphingomyelin-dependent signaling pathway with C2-ceramide. The data suggest that this oxidant-mediated apoptogenic transduction pathway may play a role in the mechanism of neuronal death in PD.
Figures
Figure 1
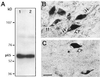
Specificity of the NF-κB-p65 antiserum. (A) Western blot of NF-κB-p65 from SNpc (lane 1) and VTA (lane 2) of a normal human mesencephalon after SDS/PAGE, blotting on nitrocellulose membranes, and immunodetection with a rabbit polyclonal antiserum (1:250 dilution) against the p65 subunit of NF-κB. After revelation by enhanced chemiluminescence, a single band at about 65 kDa was observed. NF-κB-p65 immunoreactivity is shown in neurons of normal human SNpc, with (C) and without (B) adsorption of the antiserum for 6 hr at room temperature with a 2 × 104 excess of the corresponding peptide. Open arrows, neuromelanin-containing dopaminergic neurons; solid arrows, NF-κB immunostaining in neurons and processes. (Bar = 30 μm.)
Figure 2
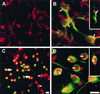
Immunohistochemical detection of NF-κB-p65 in transverse sections of control SNpc (A and B) and parkinsonian SNpc (C and D). Low- (A and C) and higher-power (B and D) photomicrographs of melanized dopaminergic neurons (large arrows) and nonmelanized neurons (arrowheads) and their processes (small arrows), with dark-blue NF-κB immunolabeling. Note in C and D neurons with strong immunoreactivity in the nucleus (open arrows), which can clearly be distinguished from the labeled perikarya (solid arrows). (Bar: A and C, 60 μm; B and D, 30 μm.)
Figure 3
ROS-induced DCDHF fluorescence and translocation of NF-κB in ceramide-treated primary cultures of mesencephalon. (A) Neurons in untreated cultures, identified by rhodamine (red) immunofluorescence with an antibody against MAP-2. (B) NF-κB in untreated cultures, identified by rhodamine (red) immunofluorescence in neurons (arrows) is preferentially localized in the cytoplasm, and appears yellow because of superimposition with fluorescein (green) MAP-2 or tyrosine hydroxylase (Inset) immunoreactivity. (C) ROS-activated DCDHF green fluorescence that appears orange/yellow/green due to superimposition on red MAP-2 staining in neurons in cultures treated with 25 μM C2-ceramide for 5 hr. (D) NF-κB (red) labeling is primarily in the nuclei (arrows) of green MAP-2 and tyrosine hydroxylase-immunolabeled neurons in ceramide-treated cultures (Inset). [Bar = 20 μm (30 μm for Insets)].
Figure 4
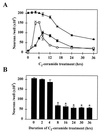
Time course of ROS-induced DCDHF fluorescence, nuclear translocation of NF-κB, and neuronal death in ceramide-treated primary cultures of mesencephalon. (A) Time course of ROS-induced DCDHF fluorescence (○), translocation of NF-κB (•), and neuronal cell death (▴) during treatment with 25 μM C2-ceramide. At each time point after the initiation of treatment, the number of cells with levels of DCDHF fluorescence (mean gray level per cell) 2 SD or more above the control level, the number of MAP-2-positive neurons with NF-κB immunoreactivity in the nucleus, and the total number of MAP-2-positive neurons were counted. (B) The numbers of MAP-2-positive neurons surviving at 36 hr were counted in cultures treated with C2-ceramide for the durations indicated: an 8-hr treatment, corresponding to the peak translocation of NF-κB in A, was both necessary and sufficient to provoke maximal neuronal death at 36 hr. Data represent the mean ± SEM of three independent experiments. ∗, Significantly different compared with untreated cultures (P < 0.0001, two-tailed t test).
Figure 5
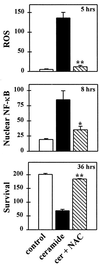
The thiol antioxidant N-acetyl-cysteine prevents ROS-induced DCDHF fluorescence, NF-κB translocation, and neuronal death induced by C2-ceramide. N-acetyl-cysteine (20 mM) was added 1 hr before and during an 8-hr exposure to C2-ceramide (25 μM), which was then withdrawn and the cells maintained in fresh N-acetyl-cysteine supplemented medium. The number of DCDHF fluorescent cells (see Fig. 4 legend) and the number of MAP-2-positive neurons with nuclear NF-κB staining were counted at their respective peaks, 5 and 8 hr after the initiation of C2-ceramide treatment (see Fig. 4_A_) and neuronal survival at 36 hr. Data represent the mean number of neurons per well (×103) ± SEM of three independent experiments. Significantly different from cultures treated with C2-ceramide alone (∗, P < 0.05; ∗∗, P < 0.01, two-tailed t test).
Figure 6
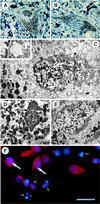
Morphological characteristics of NF-κB-positive nuclei in patients with PD and in ceramide-treated mesencephalic cultures. Photomicrographs of pigmented neurons from a parkinsonian patient with (A) and without (B) NF-κB labeling in the nucleus: semithin section counterstained with toluidine blue, on which the brown diaminobenzidine immunolabeling (arrow) could be distinguished from the black neuromelanin (arrowhead). (C) Ultrastructural analysis of nucleus in A; NF-κB immunolabeling (large arrow), dispersed chromatin (small arrow), convoluted nuclear envelope (arrowheads), autophagic vesicles (stars), neuromelanin (nm). (Inset) A higher magnification of an autophagic vesicle. (D) Apoptotic pigmented neuron (same ultrathin section as C); condensed chromatin (arrow). (E) Normal pigmented neuron with chromatin dispersed throughout the nucleus (arrows). (F) Mesencephalic cultures treated for 24 hr with 25 μM C2-ceramide, doubly labeled with an antibody against NF-κB (red fluorescence) and the DNA intercalating agent Hoechst 33258 (blue fluorescence): normal appearing nucleus containing red NF-κB immunofluorescence superimposed on the blue Hoechst fluorescence (large arrows); fragmented and condensed apoptotic nucleus without NF-κB staining (small arrows). [Bar = 30 μm (A and B), 4 μm (C and D), 800 nm (Inset in C), 5.5 μm (E), 20 μm (F).]
Similar articles
- α-Synuclein overexpression enhances manganese-induced neurotoxicity through the NF-κB-mediated pathway.
Prabhakaran K, Chapman GD, Gunasekar PG. Prabhakaran K, et al. Toxicol Mech Methods. 2011 Jul;21(6):435-43. doi: 10.3109/15376516.2011.560210. Epub 2011 Mar 21. Toxicol Mech Methods. 2011. PMID: 21417633 - [Neuronal death caused by apoptosis in Parkinson disease].
Ruberg M, France-Lanord V, Brugg B, Lambeng N, Michel PP, Anglade P, Hunot S, Damier P, Faucheux B, Hirsch E, Agid Y. Ruberg M, et al. Rev Neurol (Paris). 1997 Sep;153(8-9):499-508. Rev Neurol (Paris). 1997. PMID: 9683999 Review. French. - Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson's disease.
France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. France-Lanord V, et al. J Neurochem. 1997 Oct;69(4):1612-21. doi: 10.1046/j.1471-4159.1997.69041612.x. J Neurochem. 1997. PMID: 9326290 - NF-kappaB contributes to 6-hydroxydopamine-induced apoptosis of nigral dopaminergic neurons through p53.
Liang ZQ, Li YL, Zhao XL, Han R, Wang XX, Wang Y, Chase TN, Bennett MC, Qin ZH. Liang ZQ, et al. Brain Res. 2007 May 11;1145:190-203. doi: 10.1016/j.brainres.2007.01.130. Epub 2007 Feb 7. Brain Res. 2007. PMID: 17368433 - NF-κB-Mediated Neuroinflammation in Parkinson's Disease and Potential Therapeutic Effect of Polyphenols.
Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Singh SP. Singh SS, et al. Neurotox Res. 2020 Mar;37(3):491-507. doi: 10.1007/s12640-019-00147-2. Epub 2019 Dec 10. Neurotox Res. 2020. PMID: 31823227 Review.
Cited by
- NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease.
Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL, Lu HC, Zheng H. Lian H, et al. Neuron. 2015 Jan 7;85(1):101-115. doi: 10.1016/j.neuron.2014.11.018. Epub 2014 Dec 18. Neuron. 2015. PMID: 25533482 Free PMC article. - RING finger protein 11 (RNF11) modulates susceptibility to 6-OHDA-induced nigral degeneration and behavioral deficits through NF-κB signaling in dopaminergic cells.
Pranski EL, Dalal NV, Sanford CV, Herskowitz JH, Gearing M, Lazo C, Miller GW, Lah JJ, Levey AI, Betarbet RS. Pranski EL, et al. Neurobiol Dis. 2013 Jun;54:264-79. doi: 10.1016/j.nbd.2012.12.018. Epub 2013 Jan 11. Neurobiol Dis. 2013. PMID: 23318928 Free PMC article. - Involvement of inhibitory PAS domain protein in neuronal cell death in Parkinson's disease.
Torii S, Kasai S, Suzuki A, Todoroki Y, Yokozawa K, Yasumoto KI, Seike N, Kiyonari H, Mukumoto Y, Kakita A, Sogawa K. Torii S, et al. Cell Death Discov. 2015 Aug 17;1:15015. doi: 10.1038/cddiscovery.2015.15. eCollection 2015. Cell Death Discov. 2015. PMID: 27551449 Free PMC article. - Predicting structural features of selected flavonoids responsible for neuroprotection in a Drosophila model of Parkinson's disease.
Maitra U, Conger J, Owens MMM, Ciesla L. Maitra U, et al. Neurotoxicology. 2023 May;96:1-12. doi: 10.1016/j.neuro.2023.02.008. Epub 2023 Feb 21. Neurotoxicology. 2023. PMID: 36822376 Free PMC article.
References
- Hirsch, E. C. (1993) Eur. Neurol. 33, Suppl. 1, 52–59. - PubMed
- Mochizuki H, Goto K, Mori H, Mizuno Y. J Neurol Sci. 1996;137:120–123. - PubMed
- Anglade P, Vyas S, Javoy-Agid F, Herrero M T, Michel P P, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch E C, Agid Y. Histol Histopathol. 1997;12:25–31. - PubMed
- Dexter D T, Carter C J, Wells F R, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden C D. J Neurochem. 1989;52:381–389. - PubMed
- Saggu H, Cooksey J, Dexter D, Wells F R, Lees A, Jenner P, Marsden C D. J Neurochem. 1989;53:692–697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous