Differential glycosylation of tractin and LeechCAM, two novel Ig superfamily members, regulates neurite extension and fascicle formation - PubMed (original) (raw)
Differential glycosylation of tractin and LeechCAM, two novel Ig superfamily members, regulates neurite extension and fascicle formation
Y Huang et al. J Cell Biol. 1997.
Abstract
By immunoaffinity purification with the mAb Lan3-2, we have identified two novel Ig superfamily members, Tractin and LeechCAM. LeechCAM is an NCAM/FasII/ApCAM homologue, whereas Tractin is a cleaved protein with several unique features that include a PG/YG repeat domain that may be part of or interact with the extracellular matrix. Tractin and LeechCAM are widely expressed neural proteins that are differentially glycosylated in sets and subsets of peripheral sensory neurons that form specific fascicles in the central nervous system. In vivo antibody perturbation of the Lan3-2 glycoepitope demonstrates that it can selectively regulate extension of neurites and filopodia. Thus, these experiments provide evidence that differential glycosylation can confer functional diversity and specificity to widely expressed neural proteins.
Figures
Figure 1
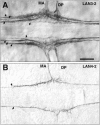
Axonal tracts labeled by the mAbs Lan3-2 and Lan4-2 in Hirudo CNS. (A) Lan3-2 labels a glycoepitope expressed by peripheral sensory neurons, the axons of which enter the CNS through the median anterior (MA) and dorsal posterior (DP) nerves and segregate into four distinct axonal tracts (arrows). The leech nervous system is bilaterally symmetrical, and anterior is to the left in this and all the following figures. (B) Lan4-2 labels a different glycoepitope expressed by a small subset of the Lan3-2–positive neurons, the axons of which segregate into a single axon tract (arrows). Bar, 25 μm.
Figure 2
Peptide sequences and new antibodies from purified Lan3-2 130-kD antigen in Haemopis. (A) Western blot of Lan3-2 immunoaffinity-purified antigen from Haemopis CNS labeled with amido black (lane 1) compared with an immunoblot of the same material labeled by Lan3-2 antibody (lane 2). The broad 130-kD band was cut out as indicated on the figure and microsequenced. Eight peptide sequences were deduced as shown to the right. (B) Western blots of Haemopis CNS proteins labeled by rabbit antisera that were made to synthetic peptides based on the sequences from pep2, pep3, and pep6 shown in bold in A. All three peptide antisera were specific to the broad 130-kD band. 200 kD is at the top of the blots in A and B, and 25 kD is at the bottom.
Figure 3
The predicted protein sequence of Tractin and LeechCAM in Hirudo. The cDNAs for Tractin (A) and LeechCAM (B) encode two novel members of the Ig superfamily. The cysteines of the Ig-like domains are indicated by arrowheads; potential N-linked glycosylation sites are indicated by stippled boxes; the hydrophobic signal sequences are underlined; and the putative transmembrane domains are double underlined. In addition, the sequences matching the deduced peptide sequences from the microsequencing of immunopurified Haemopis Lan3-2 antigen are shown in bold. Where the sequences are not an exact match, this difference could be due to a misassignment during microsequencing and/or species differences. The predicted Tractin protein sequence (A) consists of 1,880 residues or 1,855 without the signal sequence with an estimated molecular mass of 197 kD. It contains an RGD integrin binding motif (boxed), a putative RKRRSRSK peptide cleavage site (boxed), and a 60-residue highly acidic domain (stippled underline). The predicted LeechCAM sequence (B) is for a protein of 858 amino acids or 841 without signal sequence with an estimated molecular mass of 95 kD. These sequence data are available from EMBL/GenBank/DDBJ under accession numbers U92813 (Tractin) and U92814 (LeechCAM).
Figure 4
Diagrams of the Tractin and LeechCAM proteins. The protein sequence of Tractin is organized into six Ig-like domains, four FNIII-like domains, an acidic domain, a PG/YG repeat–containing domain, a transmembrane domain, and an intracellular domain with an ankyrin binding motif. The locations of the RGD integrin binding motif in the second FNIII-like domain and the putative peptide cleavage site in the third FNIII-like domain are indicated. Above the diagram of Tractin and below the LeechCAM protein sequence are partial restriction maps of the cDNAs relative to the independent, overlapping cDNA library clones from which the complete sequences were compiled (black lines). The protein sequence of LeechCAM consists of five Ig-like domains, two FNIII-like domains, a transmembrane domain, and a short intracellular tail. As indicated on the figure, the Tractin and LeechCAM sequence each matched four of the eight originally microsequenced peptides from the Lan3-2 immunopurification. In addition, the sequences used for Northern blot analysis are indicated as stippled lines below the restriction maps. Three different sequences (A, B, and C) were used to probe Tractin Northern blots.
Figure 5
The PY/GG repeat region of Tractin and sequence comparison of the ankyrin binding motif. (A) Dot plot of the Tractin amino acid sequence. The sequence was analyzed for similar sequences within itself by the computer program COMPARE with a stringency of 10 and window length of 8. Points at which the two sequences are homologous are indicated by dots. The plot reveals an extended region encompassing ∼25% of the entire amino acid sequence with an internal repeat structure. (B) A sequence alignment of the region identified in A demonstrates that it is made up of 12 repeats of a 30–amino acid motif rich in prolines and glycines in which 10 positions are invariant (asterisks). The consensus sequence for the repeats shown at the bottom is made up of 10 repetitions of the amino acid triplet PY/GG. The 12 repeat segments are connected by short linker sequences. Proline, glycine, and tyrosine residues are highlighted in white letters on a black background. (C) Sequence alignment of the ankyrin binding motif of the intracellular domain with comparable sequences from members of the L1 subfamily of CAMs: neurofascin (S26180), nrcam (P35331), L1 (P11627), ngcam (Q03696), and Drosophila neuroglian (P20241). Conserved residues of these sequences with Tractin are highlighted in white letters on a black background.
Figure 6
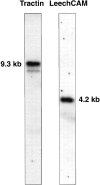
Northern blot analysis of Tractin and LeechCAM mRNA. 20 μg of total Hirudo polyA+ mRNA was fractionated on a 1.2% agarose formaldehyde denaturing gel, transferred to nitrocellulose, and probed under high stringency conditions using random primer–labeled Tractin and LeechCAM sequences. For Tractin, sequences from a 5′ end EcoRI fragment coding for the first five Ig-like domains were used, and for LeechCAM, sequences from a 5′ EcoRI fragment coding for the first three Ig-like domains were used (see Fig. 4). For Tractin, a major band at 9.3 kb and a minor band at 8.5 kb were detected, whereas a single band of 4.2 kb was observed for LeechCAM.
Figure 7
Tractin and LeechCAM are separate proteins that are both glycosylated with the Lan3-2 and Lan4-2 epitopes. (A) Immunoblots of Lan3-2–immunoprecipitated Haemopis nerve cord proteins. The immunoprecipitate was sequentially washed with 1 M NaCl, 4 M MgCl2, 3 M NaSCN, and 4 M urea before separation by 10% SDS-PAGE. A broad 130-kD band (arrow) was recognized by both Lan3-2 and Lan4-2 and by Tractin (pep2) and LeechCAM (pep6) antisera (200 kD is at the top and 25 kD is at the bottom of the blot). (B) Immunoblots of immunoaffinity-purified Lan3-2 antigen from Haemopis nerve cords separated by two-dimensional-gel electrophoresis. The purified nerve cord proteins were subjected to IEF in the first dimension (range: pH 4.5–8.5) and 7.5% SDS-PAGE in the second (200 kD is at the top and 25 kD is at the bottom of the blot). LeechCAM antiserum (B3) recognizes an oblong diffuse 130-kD spot (the arrow indicates the location of this position in all four panels) that is not labeled by the Tractin mAb 4G5 (B4). In contrast, the Tractin mAb 4G5 labels 130-kD protein in a broad range of more basic pHs (B4) that are not labeled by the LeechCAM antiserum (B3). Both Lan3-2 and Lan4-2 label proteins in a pattern matching the combined pattern of Tractin and LeechCAM antibody (B1 and B2).
Figure 8
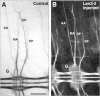
Tractin and LeechCAM are widely expressed proteins that are differentially glycosylated with the Lan3-2 and Lan4-2 epitopes in distinct subpopulations of peripheral sensory neurons. (A–D) Hemisegments of Hirudo embryos at approximately the same developmental stage (E10–11) labeled by Tractin (mAb 4G5 to pep2), LeechCAM (mouse antiserum to pep6), Lan3-2, and Lan4-2 antibody. Tractin antibody (A) labels the soma and projections of all neurons in both the central (G) and peripheral nervous system. S6 and S7 indicate the location of the dorsal sensilla, whereas some of the somata of nonsensillar peripheral neurons are indicated by arrows. LeechCAM antibody (B) labels central (G) and sensillar neurons (S6 and S7) and their projections in the four peripheral nerves (AA, MA, DP, and PP), in addition to unidentified cells in tissue forming the nephridiopore (N). Lan3-2 (C) recognizes a glycoepitope expressed on the soma and axons of all sensillar neurons. Their axons project into the CNS (G) through the MA and DP nerves where they segregate into four distinct fascicles. Lan4-2 (D) recognizes a different glycoepitope expressed by a subset of the Lan3-2–positive sensillar neurons, the axons of which segregate into a single tract in the CNS (G). Bars: (A and B) 75 μm; (C and D) 100 μm.
Figure 9
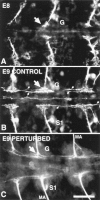
In vivo perturbation by Lan3-2 antibody of neurite extension and fascicle formation of sensillar neurons from three segments. (A) Anterior ganglia labeled by Lan3-2 antibody, showing the degree of development at the time Lan3-2 antibody or control mouse IgG1 antibody were injected into sibling embryos at E8. The location of the primordial middle ganglion is indicated by the G, and the arrow points to the choice point for segregation into fascicles of the growth cones of S3 sensillar neurons that have just entered the ganglionic neuropil. At this stage only the S3 sensillum has differentiated. (B) Uninjected control embryo from the same cocoon as the embryo in A was dissected, fixed 24 h later at E9, and labeled with Lan3-2 antibody. The control embryo shows the extensive elaboration of growth cones and filopodia at the choice point for segregation of axons into different fascicles (arrow). Several fascicles are forming (small arrows) in both the anterior and posterior direction. The most ventral sensillum (S1) has differentiated at this stage and extends axons to the CNS. (C) Sibling embryo of that shown in B injected with 0.5 μg of purified Lan3-2 antibody at E8. As in B, the embryo was dissected and fixed 24 h later at E9, and the Lan3-2 labeling was visualized with Texas red–conjugated second antibody. The axons of the sensilla in the MA nerve are still fasciculated; however, at the choice point (arrow) in the ganglia (G), the extension of growth cones and filopodia is greatly reduced and fascicle formation is truncated. Bar, 40 μm.
Figure 10
Central neurons and their projections develop normally in Lan3-2–injected embryos. (A) Hemisegment of an E9 control embryo labeled with an antibody to acetylated tubulin that shows the ganglionic nerve tracts and peripheral projections of central neurons. The figure is a Nomarski image of antibody labeling visualized with HRP-conjugated secondary antibody. (B) Hemisegment of an E9 embryo labeled with acetylated tubulin antibody that was injected with 0.5 μg of Lan3-2 antibody as in Fig. 9_C._ The organization and extent of central neuron projections is indistinguishable from that of uninjected controls (A). The figure is a fluorescence image of antibody labeling visualized with FITC-conjugated second antibody. G, location of the ganglion; AA, MA, DP, and PP; position of the four peripheral nerves. Bar, 100 μm.
Similar articles
- Differential glycosylation and proteolytical processing of LeechCAM in central and peripheral leech neurons.
Jie C, Zipser B, Jellies J, Johansen KM, Johansen J. Jie C, et al. Biochim Biophys Acta. 1999 Nov 11;1452(2):161-71. doi: 10.1016/s0167-4889(99)00118-4. Biochim Biophys Acta. 1999. PMID: 10559469 - Posttranslational processing and differential glycosylation of Tractin, an Ig-superfamily member involved in regulation of axonal outgrowth.
Jie C, Xu Y, Wang D, Lukin D, Zipser B, Jellies J, Johansen KM, Johansen J. Jie C, et al. Biochim Biophys Acta. 2000 Jun 15;1479(1-2):1-14. doi: 10.1016/s0167-4838(00)00030-3. Biochim Biophys Acta. 2000. PMID: 11004526 - Complementary expression and heterophilic interactions between IgLON family members neurotrimin and LAMP.
Gil OD, Zhang L, Chen S, Ren YQ, Pimenta A, Zanazzi G, Hillman D, Levitt P, Salzer JL. Gil OD, et al. J Neurobiol. 2002 Jun 5;51(3):190-204. doi: 10.1002/neu.10050. J Neurobiol. 2002. PMID: 11984841 - Sequential steps of carbohydrate signaling mediate sensory afferent differentiation.
Tai MH, Zipser B. Tai MH, et al. J Neurocytol. 2002 Sep-Nov;31(8-9):743-54. doi: 10.1023/a:1025756015281. J Neurocytol. 2002. PMID: 14501211 Review. - Tenascin-contactin/F11 interactions: a clue for a developmental role?
Vaughan L, Weber P, D'Alessandri L, Zisch AH, Winterhalter KH. Vaughan L, et al. Perspect Dev Neurobiol. 1994;2(1):43-52. Perspect Dev Neurobiol. 1994. PMID: 7530143 Review.
Cited by
- Multiple changes in peptide and lipid expression associated with regeneration in the nervous system of the medicinal leech.
Meriaux C, Arafah K, Tasiemski A, Wisztorski M, Bruand J, Boidin-Wichlacz C, Desmons A, Debois D, Laprévote O, Brunelle A, Gaasterland T, Macagno E, Fournier I, Salzet M. Meriaux C, et al. PLoS One. 2011 Apr 22;6(4):e18359. doi: 10.1371/journal.pone.0018359. PLoS One. 2011. PMID: 21526169 Free PMC article. - Cell adhesion molecule L1 in folded (horseshoe) and extended conformations.
Schürmann G, Haspel J, Grumet M, Erickson HP. Schürmann G, et al. Mol Biol Cell. 2001 Jun;12(6):1765-73. doi: 10.1091/mbc.12.6.1765. Mol Biol Cell. 2001. PMID: 11408583 Free PMC article. - Skeletor, a novel chromosomal protein that redistributes during mitosis provides evidence for the formation of a spindle matrix.
Walker DL, Wang D, Jin Y, Rath U, Wang Y, Johansen J, Johansen KM. Walker DL, et al. J Cell Biol. 2000 Dec 25;151(7):1401-12. doi: 10.1083/jcb.151.7.1401. J Cell Biol. 2000. PMID: 11134070 Free PMC article. - Colonization state influences the hemocyte proteome in a beneficial squid-Vibrio symbiosis.
Schleicher TR, VerBerkmoes NC, Shah M, Nyholm SV. Schleicher TR, et al. Mol Cell Proteomics. 2014 Oct;13(10):2673-86. doi: 10.1074/mcp.M113.037259. Epub 2014 Jul 18. Mol Cell Proteomics. 2014. PMID: 25038065 Free PMC article. - A phylogenetic analysis of the L1 family of neural cell adhesion molecules.
Mualla R, Nagaraj K, Hortsch M. Mualla R, et al. Neurochem Res. 2013 Jun;38(6):1196-207. doi: 10.1007/s11064-012-0892-0. Epub 2012 Sep 26. Neurochem Res. 2013. PMID: 23011207
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1987. Current Protocols in Molecular Biology. John Wiley, New York.
- Bajt ML, Cole RN, Zipser B. The specificity of the 130-kD leech sensory afferent proteins is encoded by their carbohydrate epitopes. J Neurochem. 1990;55:2117–2125. - PubMed
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. - PubMed
- Branden, C., and J. Tooze. 1991. Introduction to Protein Structure. Garland, New York. 302 pp.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous