Daxx, a novel Fas-binding protein that activates JNK and apoptosis - PubMed (original) (raw)
Daxx, a novel Fas-binding protein that activates JNK and apoptosis
X Yang et al. Cell. 1997.
Abstract
The Fas cell surface receptor induces apoptosis upon receptor oligomerization. We have identified a novel signaling protein, termed Daxx, that binds specifically to the Fas death domain. Overexpression of Daxx enhances Fas-mediated apoptosis and activates the Jun N-terminal kinase (JNK) pathway. A C-terminal portion of Daxx interacts with the Fas death domain, while a different region activates both JNK and apoptosis. The Fas-binding domain of Daxx is a dominant-negative inhibitor of both Fas-induced apoptosis and JNK activation, while the FADD death domain partially inhibits death but not JNK activation. The Daxx apoptotic pathway is sensitive to both Bcl-2 and dominant-negative JNK pathway components and acts cooperatively with the FADD pathway. Thus, Daxx and FADD define two distinct apoptotic pathways downstream of Fas.
Figures
Figure 1. Interaction of Clone A and Daxx with Fas Death Domain in Yeast
(A) Schematic representation of the cytoplasmic domain of murine Fas (Watanabe-Fukunaga et al., 1992). Boundaries of the transmembrane domain (TM), death domain (DD), and the negative regulatory domain (NR) are labeled. (B) Protein interactions in the two-hybrid system. LexA constructs contained the indicated sequences (amino acids in parentheses) of receptors that expressed similar level of fusion proteins in yeast. Colony color and β-galactosidase units were determined as described in Experimental Procedures. The activation hybrid Act-DaxxC501 contained amino acids 501–739 of Daxx. (C) The C terminus of clone A interacts with Fas death domain. Amino acids contained in each activation hybrid are indicated.
Figure 2. Daxx Sequence and mRNA Distribution
(A) Conceptually translated amino acid sequence of Daxx protein. The open reading frame of murine Daxx follows an in-frame stop codon and begins with a Kozak consensus sequence. The regions enriched for acidic residues and proline are underlined. The partial human cDNA sequence from A21 is shown below the mouse sequence with identical amino acids indicated by dashes. (B) Tissue distribution of Daxx. A mouse multiple tissue Northern blot was probed with a C-terminal 0.7 kb fragment of Daxx and a human β-actin cDNA.
Figure 3. Interaction of Daxx with Fas In Vitro and in Mammalian Cells
(A) Binding of in vitro translated 35S-Daxx to GST-fusion proteins. Positions of MW standards (in kDa) are shown at left. Coomassiestained GST fusion proteins from the same gel were aligned to show protein levels. (B) Binding of full-length and truncated 35S-Daxx to GST (lanes 3, 5, and 7) and GST-FasDD (lanes 2, 4, and 6). Daxx(–162) lacked the C-terminal 162 aa of Daxx; DaxxC corresponded to aa 628–739. Input of 35S-Daxx and 35S-Daxx(–162) proteins in binding assays are shown in lanes 1 and 2, respectively. GST fusion proteins are shown on the bottom panel. (C) Association of HA-Daxx and HA-DaxxΔC (lacking aa 626–739) with the GST fusion of Fas intracellular tail (GST-FasIC) in 293 cells (top panel). The presence of HA-Daxx and HA-DaxxΔC in extracts was verified by immunoblotting for HA (bottom panel).
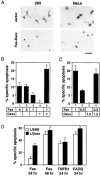
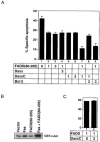
Figure 4. Daxx Potentiates Fas-Induced Apoptosis
(A) Normal and apoptotic 293 and HeLa cells. Cells were transiently transfected with the indicated plasmids, stained with X-Gal, and examined by light microscopy. Fields were chosen to illustrate morphologic differences but not relative percentages of apoptosis. Scale bar = 50 μm. (B) Daxx potentiates Fas-induced apoptosis in 293 cells. Indicated amounts (in μg) of pEBB-Fas and pEBB-HA-Daxx plasmids were cotransfected with 0.5 μg of pCMV-lacZ. Amounts of transfected DNA were equalized by adding vector DNA. The cells were stained with X-Gal 20 hr after transfection and analyzed for apoptotic morphology as described in Experimental Procedures. (C) Daxx potentiates Fas-induced apoptosis in HeLa cells. Transfection and specific apoptosis were done and measured as in 293 cells except that X-Gal staining was done 24 hr after transfection. (D) L929 cells stably overexpressing Daxx have accelerated apoptosis in response to Fas. L/EBB and L/Daxx were transfected with 1 μg of pEBB-Fas, pEBB-TNFR1, or pRK-FADD plus 0.2 μg of pCMV-lacZ. Specific apoptosis was determined as in Figure 4B at indicated time after transfection. Similar results were obtained with multiple L/Daxx lines.
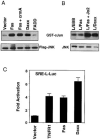
Figure 5. Daxx Activates the JNK Pathway
(A) Daxx activates JNK in transient transfection. Flag-tagged JNK1 (Flag-JNK) and the indicated plasmids (1 μg each) were cotransfected into 293 cells. Top: phosphorylation of GST-cJun. Bottom: expression of Flag-JNK. The data shown are representative of four independent assays. (B) Stable expression of Daxx constitutively activates JNK. Top: phosphorylation of GST-cJun. Bottom: expression of endogenous JNK. The data shown are representative of three independent assays. (C) Daxx activates a JNK-dependent reporter gene. The data shown are the average and SD of three independent experiments in duplicate.
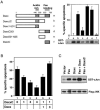
Figure 6. Deletion Analysis of Daxx
(A) Apoptosis and JNK activation by Daxx deletion mutants. The horizontal bars represent Daxx sequences present in deletion mutants. Apoptosis assay: 3 μg of each Daxx mutant construct was transfected into 293 cells as in Figure 4B. JNK assay: transient transfection of 1 μg of each Daxx mutant construct or pEBB vector (v) with 1 μg of Flag-JNK and in vitro JNK assay was doneas in Figure 5A. Equal Flag-JNK expression was verified by immunoblotting for Flag. (B) DaxxC inhibits Fas-induced apoptosis. HeLa cells were transfected with 0.5 μg pEBB-Fas and pCMV-lacZ and the indicated amount (in μg) of HA-Daxx and HA-DaxxC. Total amount of transfected DNA was made constant by adding pEBB. Jo2 antibody (12.5 ng/ml) was added 16 hr later. X-Gal staining was done 24 hr after transfection. (C) DaxxC inhibits Fas-induced JNK activation. Transient transfection of 1 μg of each indicated plasmid with 1 μg of Flag-JNK and in vitro JNK assay were done as in Figure 5A.
Figure 7. Daxx and FADD Activate Distinct Apoptotic Pathways
(A) Inhibition of Fas-induced apoptosis by DaxxC and FADD(80–205). HeLa cells were transfected with pEBB-Fas (0.5 μg), pCMV-lacZ (0.5 μg), and plasmids expressing the indicated genes (in μg). Jo2 (12.5 ng/ml) was added 16 hr later; X-Gal staining was done 24 hr after transfection. (B) FADD(80–205) fails to inhibit Fas-induced JNK activation. JNK kinase activity was assayed after transient transfection of the indicated plasmids (2 μg each) with Flag-JNK (2 μg). (C) DaxxC does not inhibit FADD-mediated apoptosis. HeLa cells were transfected with the indicated amount (in μg) of FADD and DaxxC and assayed as Figure 4C.
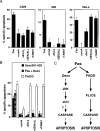
Figure 8. Inhibition Profile of Daxx- and FADD-Induced Apoptosis
(A) Inhibition profile of Fas-induced apoptosis in L929, 293, and HeLa cells. Transfection and apoptosis analysis in L/Fas cells were performed as described in Experimental Procedures. As in Figure 4B, 293 cells were cotransfected with pEBB-Fas (2 μg) plus vector or plasmids expressing indicated genes (2 μg each) and pCMV-lacZ (0.5 μg). HeLa cells were transfected with pEBB-Fas (1 μg) plus plasmids expressing the indicated genes (3 μg each) and pCMV-lacZ (0.5 μg); Jo2 (12.5 ng/ml) addition and X-Gal staining were done as in Figure 6B. (B) Inhibition of profile of Fas+Daxx, Daxx 501–625, and FADD in 293 cells. As in Figure 4B, 293 cells were transiently transfected with Fas, Daxx, Daxx 501–625, or FADD (1 μg each) plus empty vector or plasmids expressing the indicated apoptotic inhibitor genes (3 μg each) and pCMV-lacZ (0.5 μg). (C) Two pathways of Fas signaling that induce cell death.
Similar articles
- Ultraviolet radiation-induced apoptosis is mediated by Daxx.
Wu S, Loke HN, Rehemtulla A. Wu S, et al. Neoplasia. 2002 Nov-Dec;4(6):486-92. doi: 10.1038/sj.neo.7900264. Neoplasia. 2002. PMID: 12407442 Free PMC article. - Dissecting Fas signaling with an altered-specificity death-domain mutant: requirement of FADD binding for apoptosis but not Jun N-terminal kinase activation.
Chang HY, Yang X, Baltimore D. Chang HY, et al. Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1252-6. doi: 10.1073/pnas.96.4.1252. Proc Natl Acad Sci U S A. 1999. PMID: 9990010 Free PMC article. - Long form of cellular FLICE-inhibitory protein interacts with Daxx and prevents Fas-induced JNK activation.
Kim YY, Park BJ, Seo GJ, Lim JY, Lee SM, Kimm KC, Park C, Kim J, Park SI. Kim YY, et al. Biochem Biophys Res Commun. 2003 Dec 12;312(2):426-33. doi: 10.1016/j.bbrc.2003.10.144. Biochem Biophys Res Commun. 2003. PMID: 14637155 - Live and let die: regulatory mechanisms in Fas-mediated apoptosis.
Curtin JF, Cotter TG. Curtin JF, et al. Cell Signal. 2003 Nov;15(11):983-92. doi: 10.1016/s0898-6568(03)00093-7. Cell Signal. 2003. PMID: 14499341 Review. - The Daxx enigma.
Michaelson JS. Michaelson JS. Apoptosis. 2000 Jun;5(3):217-20. doi: 10.1023/a:1009696227420. Apoptosis. 2000. PMID: 11225842 Review.
Cited by
- Daxx upregulation within the cytoplasm of reovirus-infected cells is mediated by interferon and contributes to apoptosis.
Dionne KR, Zhuang Y, Leser JS, Tyler KL, Clarke P. Dionne KR, et al. J Virol. 2013 Mar;87(6):3447-60. doi: 10.1128/JVI.02324-12. Epub 2013 Jan 9. J Virol. 2013. PMID: 23302889 Free PMC article. - Tumor suppressor protein Pdcd4 interacts with Daxx and modulates the stability of Daxx and the Hipk2-dependent phosphorylation of p53 at serine 46.
Kumar N, Wethkamp N, Waters LC, Carr MD, Klempnauer KH. Kumar N, et al. Oncogenesis. 2013 Jan 14;2(1):e37. doi: 10.1038/oncsis.2012.37. Oncogenesis. 2013. PMID: 23536002 Free PMC article. - The Clinical Impact of Death Domain-Associated Protein and Holliday Junction Recognition Protein Expression in Cancer: Unmasking the Driving Forces of Neoplasia.
Pergaris A, Genaris I, Stergiou IE, Klijanienko J, Papadakos SP, Theocharis S. Pergaris A, et al. Cancers (Basel). 2023 Oct 26;15(21):5165. doi: 10.3390/cancers15215165. Cancers (Basel). 2023. PMID: 37958340 Free PMC article. Review. - Regulating Androgen Receptor Function in Prostate Cancer: Exploring the Diversity of Post-Translational Modifications.
Lumahan LEV, Arif M, Whitener AE, Yi P. Lumahan LEV, et al. Cells. 2024 Jan 19;13(2):191. doi: 10.3390/cells13020191. Cells. 2024. PMID: 38275816 Free PMC article. Review. - Mechanistic insights into the oncolytic activity of vesicular stomatitis virus in cancer immunotherapy.
Simovic B, Walsh SR, Wan Y. Simovic B, et al. Oncolytic Virother. 2015 Oct 15;4:157-67. doi: 10.2147/OV.S66079. eCollection 2015. Oncolytic Virother. 2015. PMID: 27512679 Free PMC article. Review.
References
- Abbas AK. Die and let live: eliminating dangerous lymphocytes. Cell. 1996;84:655–658. - PubMed
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor–induced cell death. Cell. 1996;85:803–815. - PubMed
- Boldin MP, Mett IL, Varfolomeev EE, Chumakov I, Shemer-Avni Y, Camonis JH, Wallach D. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/Apo1 prompts signalling for TNF and Fas/Apo1 effects. J. Biol. Chem. 1995a;270:387–391. - PubMed
- Boldin MP, Varfolomev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 1995b;270:7795–7798. - PubMed
- Brown PH, Chen TK, Birrer MJ. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene. 1994;9:791–799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous