TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes - PubMed (original) (raw)
Comparative Study
TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes
L P Sanford et al. Development. 1997 Jul.
Abstract
The growth and differentiation factor transforming growth factor-beta2 (TGFbeta2) is thought to play important roles in multiple developmental processes. Targeted disruption of the TGFbeta2 gene was undertaken to determine its essential role in vivo. TGFbeta2-null mice exhibit perinatal mortality and a wide range of developmental defects for a single gene disruption. These include cardiac, lung, craniofacial, limb, spinal column, eye, inner ear and urogenital defects. The developmental processes most commonly involved in the affected tissues include epithelial-mesenchymal interactions, cell growth, extracellular matrix production and tissue remodeling. In addition, many affected tissues have neural crest-derived components and simulate neural crest deficiencies. There is no phenotypic overlap with TGFbeta1- and TGFbeta3-null mice indicating numerous non-compensated functions between the TGFbeta isoforms.
Figures
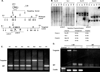
Fig. 1
Targeting of the mouse TGFβ2 gene. (A) Diagrams of the wild-type locus, the targeting vector and the predicted targeted allele. Shown is the exon structure and the primers used for various PCR reactions to distinguish the targeted from the wild-type alleles in both ES cell and tail clip DNA. Abbreviation used: B, _Bam_HI; E, _Eco_RI; H, _Hin_dIII; S, _Sac_I; X, _Xba_I; neo, poly(A)– pMC1 neo cassette. (B) Southern blots of ES cell DNA showing both control cell DNA (E14) and targeted clones. DNA was digested with the enzymes indicated and probed with either probe A or probe B. Expected restriction fragments are listed. (C) PCR-genotype of the offspring from a heterozygote (HET) intercross. The null (1.3 kb) and the wild-type (132 bp) alleles are amplified using primers p3 and p5. (D) RT-PCR analysis from HET, wild-type and null animals showing the absence of detectable TGFβ2 exon 6-specific message in the null animals. +RT and –RT represent PCR substrates produced in the presence or absence of reverse transcriptase. The β-actin lanes are PCR controls for genomic DNA contamination. The β-actin control lane is genomic DNA only.
Fig. 2
Congenital heart defects of TGFβ2-null mice. Transverse heart sections at the arterial (A,B), outflow tract (C,D) and atrioventricular valve (E,F) levels of day E18.5 of null mutant (A,C,E) and wild-type littermate (B,D,F) mice. (A) Arterial level section of normal heart showing comparable arterial wall thickness between the pulmonary trunk and the aorta. Abbreviations: a, aorta; b, bronchus; pa, pulmonary arteries; pt, pulmonary trunk; rv, right ventricle. Bar, 120 µm. (B) Arterial level section of a null heart showing a hypoplastic aorta vessel wall. Abbreviations: la, left atrium; ra, right atrium; t, trachea. Bar, 120 µm. (C) Right ventricular outflow tract level of normal heart. Abbreviation: pv, pulmonary vein. Bar, 120 µm. (D) Right ventricular outflow tract level of mutant heart showing mesenchymal septum. Abbreviation: m, myocardium of the left ventricular wall; arrow, mesenchymal outflow tract septum. Bar, 120 µm. (E) Atrioventricular valve level section of normal heart showing a normal ventricular septum. Abbreviations: lv, left ventricle; mv, mitral valve; tv, tricuspid valve. Bar, 200 µm. (F) Atrioventricular valve level section of mutant heart showing a large ventricle septum defect. Bar, 200 µm.
Fig. 3
Craniofacial defects of TGFβ2-null mice. (A) Ventral view of alcian blue (cartilage) and alizarin red (bone) staining of E18.5 skull from sibling wild-type animal. Abbreviations: a, alisphenoid; p, palatine bone; pt, pterygoid bone. Bar, 2.2 mm. (B) Ventral view of null sibling skull with cleft palate showing generally reduced ossification and the absence of the alisphenoid, pterygoid process and palatine bones. Abbreviations: f, fusion of exoccipital and basisphenoid bones; Δp, deleted palatine bone; Δpt, deleted pterygoid process. Bar, 2.2 mm. (C) Lateral view of wild-type E18.5 skull. Abbreviations: f, frontal bone; ip, interparietal bone; o, occipital bone; p, parietal bone; s, squamous bone. Bar, 2.2 mm. (D) Lateral view of null E18.5 skull showing reduced ossification of the interparietal, occipital, parietal, frontal and squamous bones. Abbreviations: Δo, deleted occipital bone. Bar, 2.2 mm. (E) Mandibles from E18.5 siblings. Abbreviation: a, angle; cp, condylar process; c, coronoid process; va, vestigial angle. Bar, 1.36 mm. (F) Lateral view of E17.5 skull from a HET animal used as a less mature growth control for A–D above. Bar, 2.2 mm. (G) Palate from a wild-type E18.5 mouse. Bar, 2.2 mm. (H) Cleft palate from a null E18.5 mouse. Bar, 2.2 mm. (I) Transverse histology section of wild-type E18.5 palate. Abbreviations: on, optic nerve; p, palate; t, tongue. Bar, 550 µm. (J) Transverse histology section of a sibling null E18.5 mouse with cleft palate showing vertical palatal shelves (ps). Bar, 550 µm.
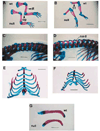
Fig. 4
Trunk and limb skeletal defects from the TGFβ2-null mice. (A) Forelimbs from E18.5 wild-type (top) and null (lower) siblings showing missing deltoid tuberosity (dt), reduced olecranon process (op) and shortened radius and ulna in null limb. Bar, 2.7 mm. (B) Hindlimbs from E18.5 wild-type (top) and null (lower) siblings showing extended foot and absent third trochanter (tt) in the mutant limb. Bar, 2.7 mm. (C) Spinal column from wild-type E18.5 mouse showing normal neural arches from a dorsal-lateral aspect. Bar, 1.4 mm. (D) Spinal column from E18.5 null sibling mouse showing the neural arch defect (nad) from a dorsal-lateral aspect. Bar, 1.4 mm. (E) Ventral rib cage from a wild-type E18.5 mouse. Bar, 2.2 mm. (F) Ventral rib cage from a null sibling E18.5 mouse showing a bifurcated sternum. Bar, 2.2 mm. (G) Clavicles from E18.5 wild-type (top) and null (lower) siblings showing ventral curvature of the null clavicle. Bar, 0.9 mm.
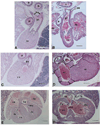
Fig. 5
Eye and inner ear defects from TGFβ2-null mice. (A) Transverse section from an E18.5 wild-type eye. Abbreviations: inl, inner neuroblastic layer; l, lens; on, optic nerve; onl, outer neuroblastic layer. Bar, 550 µm. (B) Transverse section from a null E18.5 eye showing an enlarged inner neuroblastic layer and a cellular infusion (ci). Bar, 550 µm. (C) Close-up view of the cornea from an E18.5 wild-type eye similar to the boxed region shown in A. Abbreviations: c, cornea; s, stroma. Bar, 55 µm. (D) Close-up view of the cornea from an E18.5 null mutant eye similar to the boxed region shown in B showing a reduced corneal stroma. Bar, 55 µm. (E) Toluidine blue-stained section from the basal turn of an E18.5 wild-type cochlea (right ear). Bar, 55 µm. (F) Toluidine blue-stained section from the basal turn of an E18.5 mutant cochlea (left ear) showing missing spiral limbus and interdental cells. Arrowhead indicates wider space between epithelial ridge and basilar membrane. Bar, 55 µm. Abbreviations: ger, greater epithelial ridge; idc, interdental cells; sl, spiral limbus; sv, scala vestibuli; Δsl, missing spiral limbus; rm, Reissner’s membrane; Δidc, missing interdental cells.
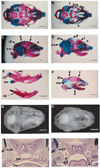
Fig. 6
Urogenital defects from TGFβ2 null mice. (A) Transverse Wilson section from a wild-type day E18.5 male showing normal genitalia. Kidneys not shown. Bar, 650 µm. (B) Transverse Wilson section from a null E18.5 male showing testicular ectopia, testicular hypoplasia and vas dysgenesis. Kidneys are present and appear normal. Bar, 650 µm. (C) Transverse Wilson section from a wild-type day E18.5 female showing normal kidney development. Bar, 300 µm. (D) Transverse Wilson section from a mutant day E18.5 female showing enlarged renal pelvis. Bar, 300 µm. (E) High-power view of dilated kidney tubules with degenerating epithelial cells and luminal protein casts. Bar, 3.7 µm. Abbreviations: b, bladder; c, colon; e, epididymis; Δe, deleted epididymis; et, ectopic testicle; ht, hypoplastic testicle; k, kidney; rp, renal pelvis.
Similar articles
- Tgfbeta2 -/- Tgfbeta3 -/- double knockout mice display severe midline fusion defects and early embryonic lethality.
Dünker N, Krieglstein K. Dünker N, et al. Anat Embryol (Berl). 2002 Dec;206(1-2):73-83. doi: 10.1007/s00429-002-0273-6. Epub 2002 Oct 1. Anat Embryol (Berl). 2002. PMID: 12478370 - Expression and action of transforming growth factor beta (TGFbeta1, TGFbeta2, TGFbeta3) in normal bovine ovarian surface epithelium and implications for human ovarian cancer.
Nilsson E, Doraiswamy V, Parrott JA, Skinner MK. Nilsson E, et al. Mol Cell Endocrinol. 2001 Sep;182(2):145-55. doi: 10.1016/s0303-7207(01)00584-6. Mol Cell Endocrinol. 2001. PMID: 11514049 - Expression patterns of Tgfbeta1-3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton.
Molin DG, Bartram U, Van der Heiden K, Van Iperen L, Speer CP, Hierck BP, Poelmann RE, Gittenberger-de-Groot AC. Molin DG, et al. Dev Dyn. 2003 Jul;227(3):431-44. doi: 10.1002/dvdy.10314. Dev Dyn. 2003. PMID: 12815630 - Transforming growth factor beta (TGFbeta) signalling in palatal growth, apoptosis and epithelial mesenchymal transformation (EMT).
Nawshad A, LaGamba D, Hay ED. Nawshad A, et al. Arch Oral Biol. 2004 Sep;49(9):675-89. doi: 10.1016/j.archoralbio.2004.05.007. Arch Oral Biol. 2004. PMID: 15275855 Review. - Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis.
Dünker N, Krieglstein K. Dünker N, et al. Eur J Biochem. 2000 Dec;267(24):6982-8. doi: 10.1046/j.1432-1327.2000.01825.x. Eur J Biochem. 2000. PMID: 11106407 Review.
Cited by
- The Role of TGF-β Signaling in Cardiomyocyte Proliferation.
Sorensen DW, van Berlo JH. Sorensen DW, et al. Curr Heart Fail Rep. 2020 Oct;17(5):225-233. doi: 10.1007/s11897-020-00470-2. Curr Heart Fail Rep. 2020. PMID: 32686010 Free PMC article. Review. - Biological Significance of Local TGF-β Activation in Liver Diseases.
Hayashi H, Sakai T. Hayashi H, et al. Front Physiol. 2012 Feb 6;3:12. doi: 10.3389/fphys.2012.00012. eCollection 2012. Front Physiol. 2012. PMID: 22363291 Free PMC article. - Targeting the TGFβ signalling pathway in disease.
Akhurst RJ, Hata A. Akhurst RJ, et al. Nat Rev Drug Discov. 2012 Oct;11(10):790-811. doi: 10.1038/nrd3810. Epub 2012 Sep 24. Nat Rev Drug Discov. 2012. PMID: 23000686 Free PMC article. Review. - TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome.
Boileau C, Guo DC, Hanna N, Regalado ES, Detaint D, Gong L, Varret M, Prakash SK, Li AH, d'Indy H, Braverman AC, Grandchamp B, Kwartler CS, Gouya L, Santos-Cortez RL, Abifadel M, Leal SM, Muti C, Shendure J, Gross MS, Rieder MJ, Vahanian A, Nickerson DA, Michel JB; National Heart, Lung, and Blood Institute (NHLBI) Go Exome Sequencing Project; Jondeau G, Milewicz DM. Boileau C, et al. Nat Genet. 2012 Jul 8;44(8):916-21. doi: 10.1038/ng.2348. Nat Genet. 2012. PMID: 22772371 Free PMC article. - Nanoceria as a possible agent for the management of COVID-19.
Allawadhi P, Khurana A, Allwadhi S, Joshi K, Packirisamy G, Bharani KK. Allawadhi P, et al. Nano Today. 2020 Dec;35:100982. doi: 10.1016/j.nantod.2020.100982. Epub 2020 Sep 15. Nano Today. 2020. PMID: 32952596 Free PMC article.
References
- Abbott BD, Birnbaum LS. Retinoic acid-induced alterations in the expression of growth factors in embryonic mouse palatal shelves. Teratology. 1990;42:597–610. - PubMed
- Barishak YR. Embryology of the eye and its adnexae. Dev. Ophthalmol. 1992;24:1–142. - PubMed
- Barnett JV, Moustakas A, Lin W, Wang XF, Lin HY, Galper JB, Maas RL. Cloning and developmental expression of the chick type II and type III TGF beta receptors. Dev. Dyn. 1994;199:12–27. - PubMed
- Besson WT, Kirby ML, Van Mierop LH, Teabeaut JR. Effects of the size of lesions of the cardiac neural crest at various embryonic ages on incidence and type of cardiac defects. Circulation. 1986;73:360–364. - PubMed
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials